AZA-1
Application:An activator of JNK and cell growth inhibitor
CAS Number:214899-21-5
Molecular Weight:842.1
Molecular Formula:C47H71NO12

The presentation will load below
Thivisha Rajagopal
Thivisha Rajagopal scored 13 on the Biological Sciences and 12 on the Physical Sciences sections of the MCAT. Thivisha has completed a B. Sc. in Medicinal Chemistry and an M.Sc. in Chemistry. Thivisha is passionate about teaching Organic Chemistry and she has been a Teaching Assistant for Organic Chemistry I and II for the past two and half years. Thivisha has also been tutoring students in General Chemistry, Organic Chemistry, and Biochemistry for over 10 years. In the classroom, Thivisha is very informal and likes to build a healthy and comfortable relationship with students. She believes it is very important to allow students to interact in discussion with their peers and the teacher.
Education
2010, M.Sc. [Chemistry]
2007, B.Sc. (Honours) [Medicinal Chemistry]
Teaching Experience
2009-Present, Lecturer, Chemistry
2009-Present, Lecturer, Biology
2008-10, Lecture TA, Organic Chemistry
2007-8, Lab TA, Organic Chemistry
1999-2010, Private Tutor, General Chemistry, Organic Chemistry, Biochemistry
Thivisha RajagopalEmail: traja085@hotmail.com
Department of Chemistry, University of Ottawa, 10 Marie Curie, Ottawa, ON, K1N 6N5, Canada
Azaspiracid-1 is an activator of JNK (c-Jun-N-terminal kinase)and caspases. It is a cellular growth inhibitor and inducer of cytoskeletal alterations. Azaspiracid-1 is also a modulator of intracellular cAMP (cyclic adenosine monophosphate) and calcium levels. It acts as an inhibitor of cholesterol biosynthesis in human T lymphocyte cells. Azaspiracid-1 is a potent teratogen to finfish and also acts as a cytotoxin to mammalian cells.
References
Multiple organ damage caused by a new toxin azaspiracid, isolated from mussels produced in Ireland: E. Ito, et al.; Toxicon 38, 917 (2000) Azaspiracid-1, a potent, nonapoptotic new phycotoxin with several cell targets: Y. Roman, et al.; Cell. Signal. 14, 703 (2002) Teratogenic effects of azaspiracid-1 identified by microinjection of Japanese medaka (Oryzias latipes) embryos: J.R. Coleman, et al.; Toxicon 45, 881 (2005) Cytotoxic and cytoskeletal effects of azaspiracid-1 on mammalian cell lines: M.J. Twiner, et al.; Toxicon 45, 891 (2005) Azaspiracids modulate intracellular pH levels in human lymphocytes: A. Alfonso, et al.; BBRC 346, 1091 (2006) Cell growth inhibition and actin cytoskeleton disorganization induced by azaspiracid-1 structure-activity studies: N. Vilarino, et al.; Chem. Res. Toxicol. 19, 1459 (2006) The c-Jun-N-terminal kinase is involved in the neurotoxic effect of azaspiracid-1: C. Vale, et al.; Cell Physiol. Biochem. 20, 957 (2007) Effects of azaspiracid-1, a potent cytotoxic agent, on primary neuronal cultures. A structure-activity relationship study: C. Vale, et al.; J. Med. Chem. 50, 356 (2007) Irreversible cytoskeletal disarrangement is independent of caspase activation during in vitro azaspiracid toxicity in human neuroblastoma cells: N. Vilarino, et al.; Biochem. Pharmacol. 74, 327 (2007) Transcriptional profiling and inhibition of cholesterol biosynthesis in human T lymphocyte cells by the marine toxin azaspiracid: M.J. Twiner, et al.; Genomics 91, 289 (2008)
Total Synthesis of (+)-Azaspiracid-1. An Exhibition of the Intricacies of Complex Molecule Synthesis
Department of Chemistry & Chemical Biology, Harvard University, Cambridge, Massachusetts 02138
J. Am. Chem. Soc., 2008, 130 (48), pp 16295–16309
DOI: 10.1021/ja804659n

The synthesis of the marine neurotoxin azaspiracid-1 has been accomplished. The individual fragments were synthesized by catalytic enantioselective processes: A hetero-Diels−Alder reaction to afford the E- and HI-ring fragments, a carbonyl-ene reaction to furnish the CD-ring fragment, and a Mukaiyama aldol reaction to deliver the FG-ring fragment. The subsequent fragment couplings were accomplished by aldol and sulfone anion methodologies. All ketalization events to form the nonacyclic target were accomplished under equilibrating conditions utilizing the imbedded configurations of the molecule to adopt one favored conformation. A final fragment coupling of the anomeric EFGHI-sulfone anion to the ABCD-aldehyde completed the convergent synthesis of (+)-azaspiracid-1.
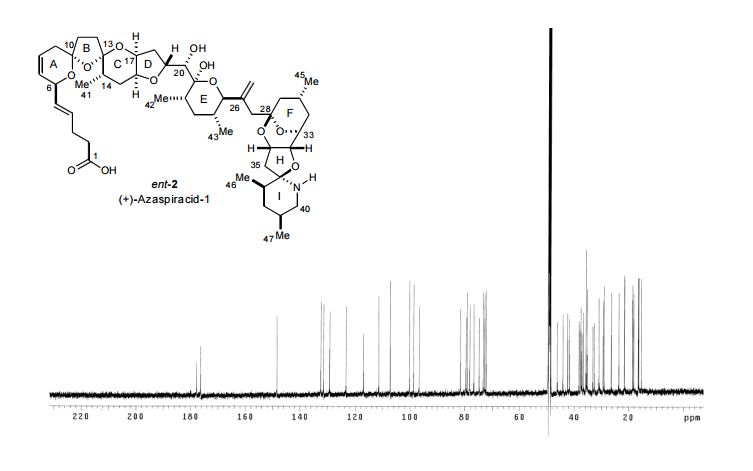
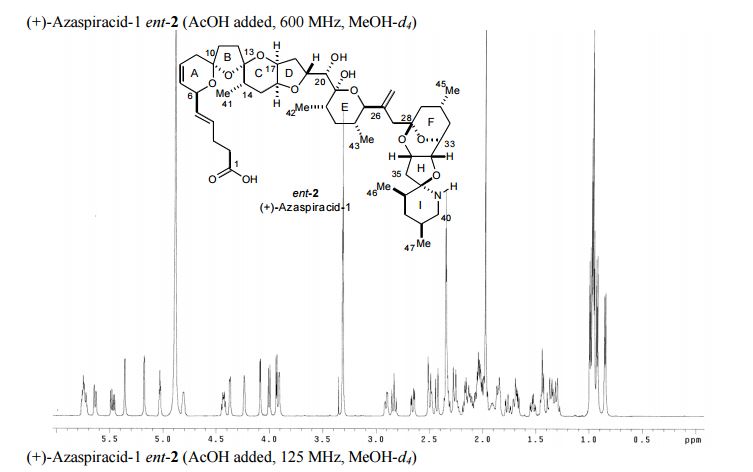
(+)-azaspiracid-1 (ent-2) (5.4 mg, 90%) as a white solid. TLC Rf = 0.25 (25:75 MeOH/EtOAc);
[α] 24 D +21.7 (c 1.00, MeOH);
IR (film) 3301, 3175, 3000 (br), 2957, 2927, 2872, 1774, 1731, 1581, 1459, 1439, 1408, 1379, 1318, 1267, 1242, 1223, 1199, 1143, 1127, 1069, 1044, 1023, 984, 875, 862, 840, 805, 734 cm−1 ;
1 H NMR (600 MHz, CD3OD, AcOH added) δ 5.78-5.71 (m, 2H, C8H, C4H), 5.64 (bd, 1H, J = 10 Hz, C7H), 5.47 (dd, 1H, J = 15, 7 Hz, C5H), 5.36 (d, 1H, J = 1 Hz, C44Ha), 5.18 (d, 1H, J = 2 Hz, C44Hb), 5.03 (t, 1H, J = 4 Hz, C34H), 4.81 (app bd, J = 2 Hz, C6H), 4.43 (td, 1H, J = 9, 6 Hz, C19H), 4.37 (bd, 1H, J = 3.5 Hz, C32H), 4.24 (bs, 1H, C17H), 4.09 (d, 1H, J = 3 Hz, C33H), 4.00 (d, 1H, J = 10 Hz, C25H), 3.93 (d, 1H, J = 5.5 Hz, C20H), 3.91 (bd, 1H, J = 2 Hz, C16H), 2.91 (bdd, 1H, J = 12, 3 Hz, C40Ha), 2.83 (t, 1H, J = 12 Hz, C40Hb), 2.66 (dd, 1H, J = 15, 4.5 Hz, C35Ha), 2.50 (d, 1H, J = 15 Hz, C35Hb), 2.51-2.47 (m, 1H, C9Ha), 2.43 (d, 1H, J = 14 Hz, C27Ha), 2.37-2.30 (m, 5H, C3H2, C11Ha, C2H2), 2.26 (d, 1H, J = 14 Hz, C27Hb), 2.27-2.22 (m, 1H, C30H), 2.19-2.09 (m, 3H, C12Ha, C9Hb, C22H), 2.09-1.95 (m, 6H, C29Ha, C14H, C18H2, C37H, C12Hb), 1.93-1.89 (m, 1H, C39H), 1.88-1.83 (m, 2H, C31Ha, C15Ha), 1.76 (app dt, 1H, J = 14, 3 Hz, C15Hb), 1.72-1.69 (m, 1H, C38Ha), 1.68 (dd, 1H, J = 12, 7 Hz, C11Hb), 1.53 (dt, 1H, J = 13.5, 5 Hz, C31Hb), 1.46-1.42 (m, 2H, C23H2), 1.40-1.27 (m, 3H, C29Hb, C24H, C38Hb), 0.99 (d, 3H, J = 7 Hz, C46H3), 0.97 (d, 6H, J = 6 Hz, C45H3, C47H3), 0.96 (d, 3H, J = 6 Hz, C41H3), 0.92 (d, 3H, J = 7 Hz, C42H3), 0.85 (d, 3H, J = 7 Hz, C43H3);
13 C NMR (125 MHz, CD3OD, AcOH added) δ 177.8 (C1), 148.4 (C26), 132.4 (C4H), 131.4 (C5H), 129.2 (C7H), 123.4 (C8H), 117.0 (C44H2), 111.3 (C13), 107.2 (C10), 100.2 (C21H), 98.7 (C28), 96.7 (C36), 81.6 (C33H), 79.6 (C25H), 79.1 (C19H), 78.2 (C16H), 76.7 (C20H), 74.8 (C34H), 73.3 (C17H), 72.8 (C32H), 72.3 (C6H), 49.2 (C27H2), 46.1 (C40H2), 44.1 (C29H2), 42.4 (C24H), 41.7 (C35H2), 38.2 (C23H2), 37.6 (C38H2), 37.5 (C12H2), 37.2 (C18H2), 36.7 (C22H), 35.7 (C9H2, C37H), 35.30, 35.25 (C2H2, C31H2), 33.2 (C11H2), 32.6 (C15H2), 30.9 (C14H), 29.3 (C3H2), 29.0 (C39H), 26.3 (C30H), 23.5 (C45H3), 18.5 (C47H3), 18.1 (C43H3), 16.6 (C41H3), 16.4 (C42H3), 15.5 (C46H3); Exact mass calcd for C47H71NO12 ([M+H] + ): 842.5054; found: 842.5023 (ESI).
http://pubs.acs.org/doi/suppl/10.1021/ja804659n/suppl_file/ja804659n_si_001.pdf
//////////Structural Elucidation, Total Synthesis , Azaspiracid-1, Thivisha Rajagopal, January 29, 2009, University of Ottawa
C[C@H]1C[C@H]2[C@@H]3[C@@H](C[C@]4(O3)[C@H](C[C@H](CN4)C)C)O[C@@](C1)(O2)CC(=C)[C@@H]5[C@H](C[C@H]([C@@](O5)([C@@H]([C@@H]6C[C@@H]7[C@H](O6)C[C@H]([C@@]8(O7)CC[C@@]9(O8)CC=C[C@H](O9)/C=C/CCC(=O)O)C)O)O)C)C


 The presentation will load below
The presentation will load below CHEMTRIX
CHEMTRIX




































 Amerikansk lovgivning holder hånden under Novozymes, når der tales om majsbaseret bioethanol, da det er lovfæstet, at 10 pct. af brændstofforbruget skal kommer fra biobrændstof
Amerikansk lovgivning holder hånden under Novozymes, når der tales om majsbaseret bioethanol, da det er lovfæstet, at 10 pct. af brændstofforbruget skal kommer fra biobrændstof






















.jpg)


