Dr D. Srinivasa Reddy of CSIR NCL @CSIR_IND wins the OPPI Scientist Award 2017 for his work in organic chemistry.
At TAJ LAND ENDS MUMBAI, INDIA
Dr D. Srinivasa Reddy of CSIR NCL @CSIR_IND wins the OPPI Scientist Award 2017 for his work in organic chemistry.
At TAJ LAND ENDS MUMBAI, INDIA
Shobha crasto receiving my Pharma excellence award for Outstanding contribution to Pharma industry from Arab health on 12 oct 2017 in Dubai, UAE..
Award at the “The Middle East Healthcare Leadership Awards” held on 12th October, 2017. At The Address,Dubai Mall – Dubai UAE….Mohammed Bin Rashid Boulevard, Downtown Dubai – Dubai – United Arab Emirates

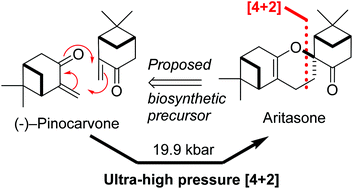
This paper describes a total synthesis of the terpene-derived natural product aritasone via the hetero-Diels–Alder [4 + 2] cyclodimerisation of pinocarvove, which represents the proposed biosyntheic route. The hetero-Diels–Alder dimerisation of pinocarvone did not proceed under standard conditions, and ultra-high pressure (19.9 kbar) was required. As it seems unlikely that these ultra-high pressures are accessible within a plant cell, we suggest that the original biosynthetic hypothesis be reconsidered, and alternatives are discussed.
Prof. Christopher Hayes began his academic career here in Nottingham with his B.Sc. in July 1992. Remaining at Nottingham, he completed his Ph.D. studies in organic chemistry, under the supervision of Professor Gerald Pattenden, in September 1995. In January 1996, on a NATO Postdoctoral Fellowship, he moved to the University of California at Berkeley where he worked in the group of Professor Clayton H. Heathcock. In September 1997, he returned to Nottingham as a Lecturer in Organic Chemistry, and has subsequently been promoted to Reader (2003), Associate Professor (2006) and Professor of Organic Chemistry (2011).
Research is centred in main-stream synthetic organic chemistry, focusing on the organic chemistry of biologically active molecules. His current research interests span a number of areas such as (i)… read more
Single crystal X-ray analysis at 100 K reveals that in the trigonal CCl4quasiracemic clathrate, space group R3, formed from host components S-(−)-Dianin’s compound and its (+)-2R,4R 2-nor methyl analogue there is an unprecedented complete ordering of a C–Cl bond of the guest with respect to the c-axial direction. In this clathrate and that formed from the (+)-2R,4R and (+)-2R,4S epimers the participation of an unexpected host conformation is reported for the first time.
Acetic acid is one of the most important bulk commodity chemicals and is currently manufactured by methanol carbonylation reactions with rhodium or iridium organometallic complexes and halide-containing promoters named Monsanto or BP Cativa™ homogeneous processes, respectively. Developing a halide-free catalyst and a heterogeneous process for methanol carbonylation is of great importance and has recently attracted extensive research attention. Here, we report a green route for direct synthesis of acetic acid via vapor-phase carbonylation of methanol with a stable, selective, halide-free, and noble metal-free catalyst based on pyridine-modified H-mordenite zeolite. Methanol conversion and acetic acid selectivity can reach up to 100% and 95%, respectively. Only little deactivation is observed during the 145 hour reaction.

A fully continuous-flow diazotization–hydrolysis protocol has been developed for the preparation of p-cresol. This process started from the diazotization of p-toluidine to form diazonium intermediate. The reaction was then quenched by urea and subsequently followed by a hydrolysis to give the final product p-cresol. Three types of byproducts were initially found in this reaction sequence. After an optimization of reaction conditions (based on impurity analysis), side reactions were eminently inhibited, and a total yield up to 91% were ultimately obtained with a productivity of 388 g/h. The continuous-flow methodology was used to avoid accumulation of the highly energetic and potentially explosive diazonium salt to realize the safe preparation for p-cresol.
. 1H NMR (400 MHz, (CD3)2SO) δ/ppm: 9.06 (br s, 1H, −OH), 6.94 (d, J = 8.0 Hz, 2H, Ar–H), 6.62 (d, J = 8.0 Hz, 2H, Ar–H), 2.17 (s, 3H, −CH3).
13C NMR (CDCl3) δ/ppm: 153.0, 129.9, 115.1, 20.5.
Literature data:(3b) 1H NMR (300 MHz, CDCl3) δ/ppm: 7.03 (d, J = 8.2 Hz, 2H), 6.73 (dd, J = 8.2, 2.0 Hz, 2H), 4.75 (s, 1H, OH), 2.27 (s, 3H, CH3).
13C NMR (CDCl3) δ/ppm: 153.2, 130.2, 115.2, 20.6.
3(b) Taniguchi, T.; Imoto, M.; Takeda, M.; Nakai, T.; Mihara, M.; Iwai, T.; Ito, T.; Mizuno, T.; Nomoto, A.; Ogawa, A. Heteroat. Chem. 2015, 26, 411– 416 DOI: 10.1002/hc.21275
http://pubs.acs.org/doi/full/10.1021/acs.oprd.7b00250
NMR PREDICT
Sustainable chemistry: how to produce better and more from less?
The International Symposium on Green Chemistry (ISGC) organized in 2013, 2015 and 2017 has gathered many senior and young talented scientists from all around the world (2200 attendees in three editions), either from academia or industry. Through outstanding conferences, communications, debates, and round tables, ISGC has been the witness of the rapid evolution of chemistry in the context of a sustainable development of our societies, not only at the scientific and industrial levels but also on education, networking and societal aspects. This critical review synthesizes the different points of view and the discussions having taken place at ISGC and gives a general picture of chemistry, including few scientific disciplines such as catalysis, processes, resource management, and environmental impact, among others, within the framework of sustainable development. This critical review, co-authored by researchers from public organizations and chemical companies (small, medium and large industrial groups) provides criteria and recommendations which, in our view, should be considered from the outset of research to accelerate the emergence of eco-designed products on the market.
Conclusions
Sustainable chemistry is the only mean to generate performant products and long lasting solutions able to generate business and profit for chemical industry. Performance is the best systemic answer for customer needs and our societies. Defining sustainable chemistry is, however, far to be an easy task because chemistry is a highly dynamic system. The sustainability of a value chain is for instance directly depending on the access to energy (and above all to its origin – coal, gas, biomass…) and on the supply of raw materials. In the current economic context, it could be not so easy to predict what will be the best source of energy or raw materials for a desired product in the future. The development of predictive tools is now essential and will represent probably one of the next scientific challenges in the coming years. During the last 20 years, utilization of renewable feedstocks in chemical processes has become a strategy of growing interest but it definitely does not guarantee the establishment of a sustainable chemistry. Indeed, in some cases, it is more sustainable to produce a chemical from a fossil carbon source using decarbonized energy than the reverse. It is very important to distinguish the carbon found in the final product from the carbon content corresponding to the energy which is required the product production (going from raw materials to manufacturing, end of life, etc.). In this area, the concept of biorefinery can help to secure developments and to minimize investments in production plant by mutualizing facilities and R&D initiatives. Cooperation with local producers can also be a valuable way to implement new bio‐based products while favouring sustainable agricultural practices. Whatever the raw materials (renewable or fossil), a complete and systemic life cycle analysis of the whole chain value (from resources to manufacturing, use and end of life) must be performed because it gives us an accurate picture of the overall economic, environmental and societal performances of a product in an application for a defined market. In general, one should never forget that sustainable chemistry should help the society to produce more and better (products). Emergence of sustainable innovations on the market takes a lot of time because chemists have to reinvent chemistry. To achieve our transition to a sustainable society, we must think differently and bring together the worlds of finance, manufacturers, researchers and public authorities. The current method of funding of research and innovation is not satisfying yet because too often based on short‐term projects and with high Technology Readiness Level. Governments have to realize that this funding method slows down, and sometime also hampers, the emergence of future sustainable innovations. Evolution of regulations with the aim of banning toxic, eco‐toxic or poor biodegradable products is an important driver for sustainable innovation. It is now seen and shared as a positive sign providing opportunities to develop systemically better solutions and allowing chemical companies advocating sustainable development and products as a must to stay in the competition. As examples, ban of CFC, replacement of chlorinated or other toxic solvents, substitution of endocrine disruptors lead to better solutions for the global benefit of our societies. Improving public perception and awareness on sustainable chemistry is on the way but more efforts will be needed in the future to definitely contribute to the emergence of eco‐ designed chemicals on the market.
Total synthesis of natural products via iridium catalysis
http://pubs.rsc.org/en/Content/ArticleLanding/2017/QO/C7QO00664K#!divAbstract
Catalysis with transition metals is a powerful synthetic tool for achieving a high degree of molecular complexity from relatively simple building blocks. Among these transition metals employed, iridium has attracted significant attention owing to its multifold roles in catalysis of various synthetically significant methodologies, and thus iridium catalysts are widely used in natural product synthesis. This review aims to comprehensively summarize recent accomplishments in total synthesis of natural products using iridium as the catalyst.


School of Chemical Engineering and Technology, North University of China, Taiyuan 030051, PR China


 Key Laboratory of Green Chemistry & Technology of Ministry of Education, College of Chemistry, Sichuan University, Chengdu
Key Laboratory of Green Chemistry & Technology of Ministry of Education, College of Chemistry, Sichuan University, Chengdu
///////////
Polycyclic heterocycles can be formed in good to excellent yields via photochemical conversion of the corresponding substituted aryl azides under irradiation with purple LEDs in a continuous flow reactor. The experimental set-up is tolerant to UV-sensitive functional groups while affording diverse carbazoles, as well as an indole and pyrrole framework, in short reaction times. The photochemical method is presumed to progress through a mechanism differing from the other methods of azide activation involving transition metal catalysis.
Methyl 9H-carbazole-2-carboxylate (9): Following the Photodecomposition Procedure A, starting from Methyl 2’-azido-[1,1’-biphenyl]-4-carboxylate, the crude mixture was purified by silica gel column chromatography (100 % hexanes → 10 % ethyl acetate in hexanes), to afford the desired product as a white solid (24.3 mg, 72 % yield). Following the Photodecomposition Procedure B, starting from Methyl 2’-azido-[1,1’- biphenyl]-4-carboxylate, the crude mixture was purified by silica gel column chromatography (100 % hexanes → 10 % ethyl acetate in hexanes), to afford the desired product as a white solid (27.7 mg, 82 % yield). NMR data was in accordance with what was previously reported.16
16 Takamatsu, K.; Hirano, K.; Satoh, T.; Miura, M. Org. Lett. 2014, 16, 2892-2895
4-Isopropyl-9H-carbazole (14): Following the Photodecomposition Procedure A, starting from 2-azido-2’-isopropyl-1,1’-biphenyl, the crude mixture was purified by silica gel column chromatography (100 % hexanes → 10 % ethyl acetate in hexanes), to afford the desired product as a yellow solid (16.6 mg, 53 % yield). Following the Photodecomposition Procedure B, starting from 2-azido-2’-isopropyl-1,1’-biphenyl and using ethyl acetate as the solvant, the crude mixture was purified by silica gel column chromatography (100 % hexanes → 10 % ethyl acetate in hexanes), to afford the desired product as a yellow solid (16.0 mg, 51 % yield).
1H NMR (400 MHz, DMSO-d6) δ = 11.29 (s, 1H), 8.11 (d, J = 8.1 Hz, 1H), 7.50 (d, J = 8.1 Hz, 1H), 7.39-7.31 (m, 3H), 7.19- 7.15 (m, 1H), 7.08-7.03 (m, 1H), 3.92-3.82 (m, 1H), 1.41 (d, J = 6.8 Hz, 6H);
13C NMR (100 MHz, DMSO-d6) δ = 143.9, 140.3, 140.1, 126.0, 125.2, 122.8, 122.2, 119.9, 119.1, 114.9, 111.2, 108.9, 30.2, 22.8 (2C);
HRMS (ESI) m/z calculated for C15H15N [M-H]- 208.1130; found 208.1126.
///////////http://pubs.rsc.org/en/Content/ArticleLanding/2017/GC/C7GC02261A?utm_source=feedburner&utm_medium=feed&utm_campaign=Feed%3A+rss%2FGC+%28RSC+-+Green+Chem.+latest+articles%29#!divAbstract
Catalyst-free multi-component cascade C-H-functionalization in water using molecular oxygen: an approach to 1,3-oxazines
Herein, catalyst-free 3-component reactions of naphthols, aldehydes, and tetrahydroisoquinolines to synthesize 1,3-oxazines is reported. The reaction is performed in H2O in the presence of O2 as the sole oxidant at 100 °C, which proceeds through the formation of 1-aminoalkyl-2-naphthols followed by selective α-C–H functionalization of tert-amine.
15-phenyl-7a,12,13,15-tetrahydronaphtho[1′,2′:5,6][1,3]oxazino[2,3- a]isoquinoline (4a):1
White solid; Yield 61 %, 221 mg;
1H NMR (500 MHz, CDCl3): δ 7.79-7.77 (m, 1H), 7.74 (d, J = 8.9 Hz, 1H), 7.43-7.41 (m, 1H), 7.33-7.28 (m, 8H), 7.24-7.19 (m, 3H), 7.11 (d, J = 8.9 Hz, 1H), 5.65 (s, 1H), 5.44 (s, 1H), 3.40-3.26 (m, 2H), 3.12-3.09 (m, 1H), 2.90- 2.86 (m, 1H);
13C NMR (125 MHz, CDCl3): δ 151.9, 142.3, 135.0, 133.0, 132.4, 129.3, 129.1, 128.9, 128.8 (2C), 128.7, 128.6, 128.2, 127.4, 126.5, 126.2, 123.1, 122.7, 118.9, 110.9, 82.2, 62.6, 45.4, 29.4;
HRMS (ESI) exact mass calculated for C26H21NO [M+H]+ : 364.1701; found: 364.1705.
The representative procedure for the synthesis of 4a is as follows: 2-naphthol (1a, 144 mg, 1 mmol), benzaldehyde (2a, 106 mg, 1 mmol), tetrahydroisoquinoline (3, 133 mg, 1 mmol) and water (1.5 mL) were added in a round-bottom flask equipped with a magnetic stirring bar and a reflux condenser. The whole apparatus was efficiently flushed with oxygen gas and then connected to a balloon filled with oxygen. After vigorous stirring at 100 oC for 12 h, water was removed under vacuum and purified the reaction mixture by column chromatography (100-200 mesh silica gel, hexane-ethyl acetate) to obtain the product 4a as white solid. The other 1,3-oxazines were synthesized and purified by following the procedure described above
//////////////