Scaleup of API/Drugs
As the development of a new drug product progresses, the batch sizes manufactured generally increase. The early First Time in Man (FTIM) clinical trials commonly involve dosing to a small number of subjects, with the majority of the manufactured product often being required for stability studies and analytical testing. As one proceeds from formulation design to Phase I clinical trials and subsequently through to Phase II & III, batch sizes generally increase. Following a successful clinical trial outcome, resulting in the granting of a marketing authorization, the batch sizes manufactured may be further increased to cover the market demands.
“ALL FOR DRUGS” CATERS TO EDUCATION GLOBALLY, No commercial exploits are done or advertisements added by me. This article is a compilation for educational purposes only.
P.S. : The views expressed are my personal and in no-way suggest the views of the professional body or the company that I represent

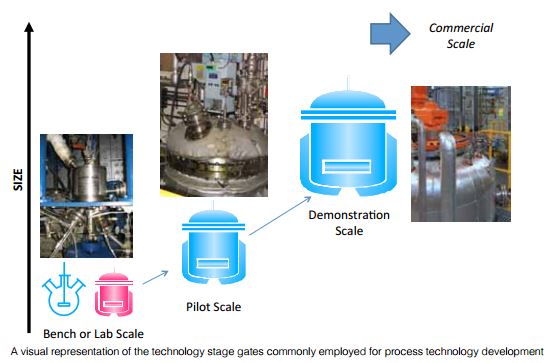
Current International Conference on Harmonization (ICH) guidelines state that data from stability studies should be provided on at least three primary batches of the drug product (1). The primary batches should be of the same formulation and packaged in the same container-closure system as proposed for the marketed formulation. The manufacturing process used for these primary batches should simulate that to be applied to production batches and should provide product of the same quality and meeting the same specification as that intended for marketing. Two of the three batches should be at least pilot-scale batches and the third one can be smaller, if justified. Where possible, batches of the drug product should be manufactured by using different batches of the drug substance. Typical batch sizes used throughout development as defined in the European Medicines Agency’s (EMA) guidance for Process Validation are laboratory-scale, pilot, and production-scale batches (2).

Laboratory-scale batches. These are produced at the research and early development laboratory stage. They may be of very small size (e.g., 100–1000 times less than production scale). Laboratory-scale batches may be used to support formulation and packaging development, early clinical and/or preclinical stages. Laboratory-scale batches can also be analyzed to assist in the evaluation and definition of critical quality attributes (CQAs). A CQA is a physical, chemical, biological, or microbiological property or characteristic that should be within an appropriate limit, range, or distribution to ensure the desired product quality. CQAs are generally associated with the drug substance, excipients, intermediates, and drug product.

Pilot-scale batches. These may be used in the process-development or optimization stage. They may be used to support preclinical and mid- to later-stage clinical evaluation and also to support formal stability studies. If supporting formal registration, a pilot-batch size should correspond to at least 10% of the production-scale batch. For oral solid-dosage forms, this size should generally be 10% of production scale or 100,000 units, whichever is greater. The choice of pilot scale is often difficult for the project team as members must balance parameters such as anticipated product volumes, anticipated site of production, equipment constraints at that site, and regulatory expectations. With the increasing trend toward developing orphan drugs, the authors believe that the regulatory expectation of pilot-scale batches of 100,000 units is not always valid and should be discussed with the relevant regulatory authority.

Production-scale batches. These batches are of the size that will be produced during the routine manufacturing and marketing of the product. Data on production-scale batches may not always be available prior to granting marketing authorization.
Approaches to process optimization and scale-up
Pharmaceutical development activities around the scaling-up and optimizing for pilot- or commercial-scale manufacture should include, at a minimum, the following elements (3):
- Defining the target product profile as it relates to quality, safety and efficacy, considering, for example, the route of administration, dosage form, bioavailability, dosage, and stability
- Identifying CQAs of the drug product, so that those product characteristics having an impact on product quality can be studied and controlled
- Determining the quality attributes of the drug substance and excipients, and selecting the type and amount of excipients to deliver drug product of the desired quality and efficacy
- Selecting an appropriate manufacturing process
- Where possible, identifying a control strategy.
The basis of quality-by-design (QbD) follows that well-designed experiments, carefully planned and thought out from the outset of formulation development, can produce quality data from an early stage, which can minimize lengthy and costly repetition of manufacturing and subsequent studies and analysis. Nevertheless, before manufacturing registration batches, it is usual to invest additional time and effort in challenging, and, where necessary, optimizing the existing manufacturing process. This is achieved by making additional development batches at laboratory-scale, pilot scale, and possibly even at production scale. The output from the above development efforts provides the basis for justification that scale up can be achieved without a consequent loss in quality and/or efficacy and provides the link between formulation and process development, pilot-scale manufacture, and eventual commercial production.
Analysis of the data assists in determining the parameters that have a known effect upon a CQA of the drug product. These parameters are referred to as being critical process parameters (CPPs). Furthermore, data obtained creates a picture of the product and process, how its design has evolved, what is critical and must be controlled, and proven acceptable ranges (PARs) around specific process parameters.
This article presents three approaches that a CDMO can consider in designing development and process optimization studies that will provide useful data on scaling up a product.

Approach 1: Quality ensured by end-product testing
This method of manufacturing is a minimal approach to the mechanistic understanding of a process. The development is mainly empirical with research often conducted one factor at a time (OFAT). Following manufacture, there would be little or no ‘design space’ defined, resulting in a strict manufacturing process that should be adhered to in order to minimize any variability in product characteristics and thus the CQA profile. Validation will primarily be based on initial full-scale batches. CPPs are determined using only prior knowledge. Proven acceptable ranges for CPPs are not fully determined. A minimal knowledge of PARs is determined. The following is an example of how this approach is used in developing a film-coated tablet.
Example 1: Blend, direct compression, and coating scale-up operation. A blending operation at a specified scale is performed using a standard tumble blender. The drive unit is set to a fixed speed. The excipients are added to the blending shell and tumbling is initiated. The blend is mixed for a set period of time. Following this mixing time, the blend is sampled to determine blend uniformity and bulk assay values. If the blend is determined to be uniform, the time and speed used is recorded and would be used for future batches. If the blend is found to be nonuniform at this stage, an additional mixing period is used, followed by further sampling for uniformity and bulk assay. This procedure is repeated until uniformity is achieved with the mixing time and speed recorded for future batches.

The uniform blend is compressed using a suitable tablet press for the batch size in question. The compression parameters are adjusted in order to obtain the desired product characteristics such as tablet weight, hardness, thickness, disintegration time, and dissolution time. Compression force and compression speed would be adjusted during set-ups and continually assessed with appropriate adjustments made. Intensive in process checks continuously monitor product quality characteristics in order to maintain an acceptable product.
The tablet cores are progressed for coating using a batch coating process. The coating parameters are initially set using prior knowledge of the equipment being used and coating at a particular batch size. Intensive in-process checks, in addition to close visual observations by trained operators, allow adjustments to be made throughout the coating process. The final parameters would be recorded and used for future coating operations at this scale.
With the advent of the guideline ICH Q8 Pharmaceutical Development, this historical approach is falling out of favor, and would likely invite greater scrutiny from regulatory authorities following submission (3).
Approach 2: Full design of experiments
An enhanced QbD approach to product development would additionally include a systematic evaluation, understanding, and refining of the formulation and manufacturing process, including (3):
- Identifying–using prior knowledge, experimentation, and risk assessment–the material attributes and process parameters that can have an effect on product CQAs
- Determining the functional relationships that link material attributes and process parameters to product CQAs
- Risk-assessment tools can be used to identify and rank potential parameters deemed to have an impact on product quality based on prior knowledge and initial experimental data. The initial list of all possible parameters can be quite extensive, but is likely to be narrowed, as process understanding is increased, to a smaller list of potential parameters. Narrowing the list has the advantage of reducing the number of experiments necessary in the modeling of a design space. The list can be refined further through screening experimentation to determine the significance of individual parameters and potential interactions. Once the significant parameters are identified, they can be further studied (e.g., through a combination of design of experiments, mathematical models, or studies that lead to mechanistic understanding) to achieve a higher level of process understanding.

Figure 1: An Ishikawa (fishbone) diagram that identifies all potential parameters that can have an impact on the desired quality attribute.
Example 2: Full design of experiments.Using prior scientific knowledge, the project team experts will use the target product profile to establish CQAs, and, in turn, CPPs. An approach could follow the five steps listed below.

Table I: The potential parameters in an example analysis.

Figure 2: Example C&E diagram detailing dry-mixing and granulation parameters.
Step 1: Cause and effect analysis (Ishikawa diagram). The expert team compiles a cause and effect (C&E) diagram, also known as an Ishikawa diagram, that maps out all potential parameters in a manufacturing process. The number of parameters can be very extensive. An example analysis performed for a recent project at Almac resulted in 79 parameters, possibly influencing the final tablet quality (see Figure 1). The parameters determined are shown in Table I. Figure 2 shows the potential CPPs making up the dry granulation step of the manufacturing process.
Step 2: Ranking of process parameters in order of importance. The list was analyzed using a risk-assessment approach to prioritize the parameters. Parameters graded as high risk were progressed for investigation. An example parameter rating form is presented in Figure 3. The example shows a parameter rating form focusing on the nine parameters from the dry-granulation stage that may have an effect on the CQAs. A separate rating form would be completed for each stage of the manufacturing process. A scoring system, ranging from 1 being minimal effect, 3 being moderate, and 9 having a major effect on product attributes, was used. This scoring approach applies a much greater weighting to parameters deemed to impact a product’s attributes significantly, thus increasing the necessity to further study the effect of the particular variable.

Figure 3: Example parameter rating form.
Figure 4 shows an example of results following the compilation of the scoring process. The example illustrated focuses on a single product attribute (tablet hardness) and a single processing step (dry granulation). The full exercise produces individual tables compiling project team views regarding each processing parameter in relation to each CQA.

Figure 4: Example results parameter rating form.

Figure 5: Parameter evalution to determine which parameters should be investigated.
Figure 5 shows how decisions can be made on which parameters may be investigated at the next stage. The total score is the sum of the scores obtained for process parameters across all the CQAs. This will provide a rank order of parameters that may have an effect on CQAs. Using prior experience coupled with knowledge of the intended process, the number of parameters can be minimized. The number of parameters investigated using experimental design will significantly increase the number of batches required.
Figure 5: Parameter evalution to determine which parameters should be investigated.
Step 3: Assessing the impact of parameters on the product. The parameters were further screened by considering the higher ranked parameters in each processing step as having a high influence on each CQA. Also, every parameter having a score of 40 or more was classified as having a high influence on CQAs. An example matrix showing the impact assessment for the dry granulation step is presented in Figure 6.

Figure 6: Detail of parameter risk assessment.
The parameters were investigated using a 2-level screening design of experiments. In this example, 10 parameters were chosen to be screened.Step 4: Determine the parameters to be investigated experimentally. Following a full analysis using prior knowledge and experience of the formulation and the manufacturing techniques, conclusions will be drawn from the risk-based classification process to list the parameters to be investigated using a first-screening experimental design (see Table II).

Table II: Parameters to be investigated in example analysis
The number of parameters, in addition to the desired resolution of the design, impacts on the number of experiments required in determining the critical parameters. Figure 7 shows the relationship between number of parameters and resolution with number of experiments required. Additional considerations such as time and active pharmaceutical ingredient constraints may also influence the number of experiments that are possible at this stage.

Figure 7: The relationship between the number of parameters and resolution of design to the number of experiments required.
| . |
The standard experimental design notation is presented in Figure 8, where X is the number of levels being investigated. A level is a particular parameter value. In the case of compression speed, the two levels could be the maximum and minimum turret speed that would potentially be used in an operation. K is the number of parameters being investigated. P is the degree of fractioning that is being performed in order to reduce the number of experiments. A full factorial design would not have a value for p displayed.

Figure 8: Standard experimental design notation
For example, 25 would be a two-level design covering 5 parameters. This would require 32 experiments to investigate the parameter effects. 25-1 is a fractional design of this example where the number of experiments required is halved to 16. As the degree of fractioning increases the quality of information obtained from the design decreases. A typical approach would be to use a high-quality (high resolution) design for optimization and in-depth study of a process and use a low-resolution design for initial screening in order to determine critical parameters. The design would then generate a number of experiments that detail the exact parameters to set for a manufacturing operation. Each experiment would be completed and the subsequent product attributes would be determined. Statistical software is used to analyze the effect of the process parameters on the product attributes and determine how critical each parameter is.
Step 5: Design space. The linkage between the process inputs (input parameters and process parameters) and the CQAs can be described in the design space. The risk-assessment and process-development experiments described can not only lead to an understanding of the linkage and effect of process inputs on product CQAs, but also help identify the parameters and their ranges within which consistent quality product can be achieved.In the described example, the results of the experimental design allowed the number of potential critical parameters for the initial screening to be reduced to eight. These eight parameters were then investigated using a more comprehensive 3-level design. This type of design, also referred to as a response surface method (RSM), will require more experiments. The previous 2-level screening design is used to minimize the number of parameters investigated so as to minimize the number of experiments at the 3-level design stage. In this example, the results of the 3-level design enabled statistically significant models to be calculated showing the effects of the critical parameters on a number of product attributes. A graphical example of a model displaying the influence of granulator final screen size and roller pressure on tablet hardness is shown in Figure 9. As can be seen, as the roller pressure and granulating screen size decreases, the tablet hardness increases.

Figure 9: The influence of granulator final screen size and roller pressure on tablet hardness.
Approach 3: Hybrid method combining elements of approaches 1 and 2.
The use of a full design of experiments approach can result in a significant amount of batches being manufactured to cover the design space. As the number of batches required to describe the design space increases, the development time and cost increases. A third method combining elements from approaches 1 and 2 optimizes the balance between development time and cost and maintains a good understanding of the manufacturing process. This hybrid approach can be achieved by breaking the manufacturing process into defined sections. The initial blending step can be performed using Approach 1. The blend could be manufactured to specification, and end-product testing will show the quality (e.g. blend uniformity/assay). Parameters such as blending time and blending speed can be determined by intensive blend uniformity and bulk-assay analysis allowing blending windows to be established. A second confirmatory batch could be manufactured using the parameters obtained from the initial batch that could provide additional confidence in the manufacturing process.
The blend will have been shown to meet specification and can then be progressed to the next processing steps. An experimental design approach can then be performed. For example, the next steps could involve dry granulation followed by compression into tablets. As described in approach 2, following completion of a full risk assessment and subsequent cause and effect diagram detailing the potential process parameters, a structured design of experiments plan can be devised. Rather than completing each experiment at the full scale, the experiment can be performed using a suitably sized aliquot of the blend manufactured already. As both dry granulation and compression are a continuous throughput process, the parameters investigated are mainly independent of batch size used. Using this approach, a 60-kg blend could be divided into 3-kg sublots, allowing up to 20 experiments to be run.
Following completion of the experiments and analysis of the data, a confirmatory batch could be manufactured using the optimized parameters determined from statistical analysis and preparation of a design space. The resulting batch or tablet cores could be progressed to coating if required. Again, the coating process can be investigated using method 1 or 2.
Conclusion
Strategies for product development can vary from company to company and from product to product, and as such, a contract development and manufacturing organization (CDMO) must be able to provide a service that can suit the requirements of any customer. For a variety of reasons, a company might choose either an empirical approach or a more systematic approach to product development. The approaches described can aid in the provision of a high-quality development offering from the outset while striving to control timelines and costs, which are commonly seen as opposing forces in the pharmaceutical industry.
Slides STUDENTS CAN USE FOR PRESENTATIONS







References
1. ICH, Q1A (R2) Stability Testing of New Drug Substances and Products, (ICH, Geneva, Feb. 2003).
2. EMA, Note for guidance, Process Validation, CPMP/QWP/848/96, (EMA, London, Sept. 2001).
3. ICH, Q8 (R2) Pharmaceutical Development, (ICH, Geneva, Aug, 2009).
CASE STUDY1




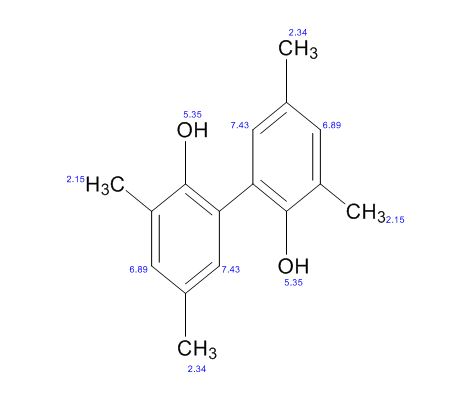
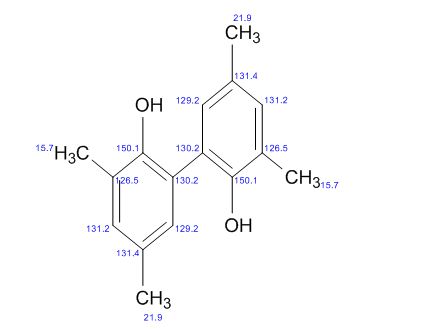
3,3′,5,5′-Tetramethyl-2,2′-biphenol
- 2,2′-Biphenyldiol, 3,3′,5,5′-tetramethyl- (8CI)
- 6,6′-Bi-2,4-xylenol (6CI,7CI)
- 3,3′,5,5′-Tetramethyl[1,1′-biphenyl]-2,2′-diol
- 2,2′-Dihydroxy-3,3′,5,5′-tetramethylbiphenyl
- 3,3′,5,5′-Tetramethyl-2,2′-biphenol
- 3,3′,5,5′-Tetramethyl-[1,1′-biphenyl]-2,2′-diol
- 3,3′,5,5′-Tetramethylbiphenyl-2,2′-diol
Scalable and Selective Preparation of 3,3′,5,5′-Tetramethyl-2,2′-biphenol

PROCESS OPTIMISATION AND SCALE-UP – CASE STUDY
Endeavour is specialised in the handling and use of a range of hazardous and often highly odorous compounds and since our acquisition by Robinson Brothers in 2008 and the successful implementation of new technology transfer processes, we are now able to offer our customers quantities ranging from a few grammes through to thousands of Kilograms.
The following case study provides an excellent example of how the technology transfer process works in practice and also highlights Endeavour-Robinson Brothers ability to rapidly scale a product from laboratory to plant.
Introduction
As part of Endeavour’s commitment to new product development, the intermediates division undertook synthesis of a variety of thioamides. These molecules are of interest as they can be used in the synthesis of hydrogen-sulphide releasing drugs (e.g. ATB-346 – a Naproxen analogue, Scheme 1).

Scheme 1: Retrosynthesis of ATB-346
They can also be used to make thiazoles via reaction with -haloketones – the Hantzsch reaction (e.g. in the synthesis of Febuxostat – an oral non-purine selective xanthine oxidase inhibitor, Scheme 2).

Scheme 2: An example of the Hantzsch reaction – the synthesis of Febuxostat
Thioamides are normally synthesised using hydrogen sulphide – a highly odorous and toxic gas. This product group was an ideal fit for Endeavour, not only because of its commercial interest, but also because Endeavour is already equipped with an efficient containment facility that removes 99.9% of all harmful and/or malodorous compounds.
The project
In mid-2008, Endeavour was approached by a blue-chip global pharmaceutical company to supply 2,2-Dimethylthiopropionamide (Figure 1) – a compound which was already part of their existing product range.

Figure 1: 2,2-Dimethylthiopropionamide structure
The initial enquiry was for 5 kg with a strict lead time. Previously, Endeavour had only furnished this compound on research (gram) scale; however, using in-house process development expertise, the required compound was delivered on time and in full, meeting all customer criteria.
Further process improvements enabled Endeavour to offer a further 8 kg in half the original lead time, which was furnished in early 2009. In late 2009/early 2010, two further orders of 45 kg and 80 kg were required but the scale of these enquiries, coupled with the stringent customer lead time, meant Endeavour could not fulfil the orders directly.
Technology transfer
Endeavour’s parent company, Robinson Brothers Limited, acquired Endeavour in April 2008. Robinson Brothers has significant expertise in scale-up from 10 kg to multi-tonnes and crucially has its own containment facility for highly odorous and toxic compounds. This allowed the project to be transferred to Robinson Brothers to fulfil the 45 kg and 80 kg orders in the desired time-frame.
The chemistry was seamlessly scaled to pilot-scale and both quality and yield were improved through further optimisation of the process. As the customer’s product trials progressed and the finished drug continued to show great promise, it became clear that the chances of commercial launch were increasing and this lead to calls for an even tighter specification than the original (>98%) purity
In early 2011, the customer requested specification revisions to both the assay (product now had to be >99.5% assay) and the sulphur content (level had to be below 0.2%). These specification revisions were then to be incorporated into a further 120 kg order for delivery in mid-2011. Thanks to the skill of Robinson Brothers’ process development team, the necessary changes were made to the reaction scheme and the finished product was delivered on-time and in full.
Future
Currently, the drug, which 2,2-dimethylthiopropionamide is used for, is in late-stage clinical trials with a view to full-scale manufacturing in the near future.
Conclusions
Few companies in Europe are capable of handling Hydrogen Sulphide on both a laboratory and commercial scale. With Endeavour-Robinson Brothers high-efficacy containment systems, a range of hazardous chemical processes are possible from gram to multi-Kilo scale.
Endeavour-Robinson Brothers can offer customers access to both an extremely diverse set of technologies and chemistries and extensive experience of process development and optimisation. With our high standards of customer service and over 140 years of successful project completion, Contact us today to find out how we can make your next project our latest success.
CASE STUDY 3

SYNTHESIS AND SCALE UP OF THE TISSUE SELECTIVE ESTROGENE ZK 186619
DESCRIPTION
Johannes Platzek spoke about the synthesis and scale up of the tissue selective Estrogene ZK 186619 (1) carried out at Schering AG. The medicinal chemistry route (see Scheme 1), 19 steps including 2 chromatographic purifications, had been successfully used to prepare 100g of product with an overall yield of 5%, but a more efficient route was required. Various scale up issues were identified – use of PCl5/SnCl4 for the cyclisation, demethylation with pyridine HCl requires temperature of 180oC, 5 equivalents of 4-methoxyphenylmagnesium bromide were required for the Grignard reaction, demethylation with NaSCH3 generates methane thiol as a by-product, and finally there were several protecting group changes in the synthesis.

Scheme 1
An alternative synthetic route was devised where the phenyl group was introduced prior to the cyclisation stage. The same starting material, 3-methoxybenzaldehyde, was used but in the new route was reacted with acetaldehyde to give 3-methoxycinnamaldehyde. The literature yield for this reaction was 10-20%, but initial optimisation studies improved this to 35% and then automated optimisation using a statistical approach gave a fully optimised yield of 80%. The intermediate 3-methoxycinnamaldehyde was not isolate but was reacted directly with phenylacetic acid to give a diene-acid intermediate which was isolated and then hydrogenated to give the cyclisation precursor. Cyclisation was effected by treatment with polyphosphoric acid (PPA), but the work-up of this reaction had to be modified for scale up. Quenching by addition of water was extremely exothermic, but the reaction mixture was too viscous to add to water. The answer was to cool the reaction mixture to 50oC, add 15% methanol to give a pumpable mixture, which could be quenched onto water.

Various methods of introducing the 4-methoxyphenyl group were investigated; including Grignard addition, aryl lithium addition, but the best method proved to be a Suzuki coupling with the enol sulphonate derived from the cycloheptanone. Some convergence was introduced to the synthetic route at this point by introducing the side chain in one piece (see Scheme 2).

A number of reagent combinations were evaluated for demethylation to give the phenol (48% HBr, HBr/HOAc, TMSCl/NaI/acetonitrile, AlCl3, BBr3/CH2Cl2) with boron tribromide/base working best. The nature of the base was critical to the success of the reaction. When pyridine was used a yield of 40% was obtained, but utilising a more hindered base, 2,6-lutidine gave much improved yield (85%), but use of an even more hindered base, 2,6-di-tert-butylpyridine was ineffective.
Introduction of the pentafluoro side chain was fairly straightforward although two routes were considered – introduction of the sulphide and then oxidation to the sulphoxide, or introduction of the side chain in one unit as the sulphoxide. This latter approach although introducing more convergence to the synthesis was less economic because of the cost of the 4,4,5,5,5-pentafluoropentan-1-ol used as starting material for this leg of the synthesis (the increased convergence meant this material had to be processed through more chemical steps). The final stages were therefore essentially as carried out in the medicinal chemistry synthesis – conversion of pentafluoropentanol in to the tosylate followed by displacement of the tosylate group with potassium thioacetate to give pentafluoropropyl thioacetate.
The demethylated chloro intermediate from the Suzuki coupling was converted in to the iodide in a Finklestein reaction in 2-butanone and this was then reacted with the thioacetate ester using sodium methoxide as base. The final oxidation was carried out with sodium metaperiodate. Hydrogen peroxide could also be used as the oxidant, but this gave a product with a poorer impurity profile.

Scheme 3
In summary the process route required 12 chemical steps (7 isolated steps) in 25% overall yield (lab, 20% on pilot plant scale) with no chromatography required.
CASE STUDY 4

https://www.vapourtec.com/flow-chemistry/process-intensification/
Development of a continuous flow scale-up approach to reflux inhibitor AZD6906
· Tomas Gustafsson
· Henrik Sörensen
· Fritiof Pontén
Early scale-up work of a promising reflux inhibitor AZD6906 is described. Two steps of an earlier route were adapted to be performed in continuous flow to avoid issues related to batch procedures, resulting in a robust method with reduced cost of goods and improved product quality. Toxic and reactive reagents and starting materials could be handled in a flow regime, thereby allowing safer and more convenient reaction optimization and production.

///////////3,3′,5,5′-Tetramethyl-2,2′-biphenol, scaleup
“ALL FOR DRUGS” CATERS TO EDUCATION GLOBALLY, No commercial exploits are done or advertisements added by me. This article is a compilation for educational purposes only.
P.S. : The views expressed are my personal and in no-way suggest the views of the professional body or the company that I represent
Technology Transfer failures that are easily avoided
The failure of a Technology Transfer process will, at best result in delays to product sales and increased costs, and at worst may lead to 483 notices, warning letters, loss of reputation and most importantly an impact on the health of potential recipients of the treatment.
During my observation of Technology Transfer projects, the following reasons rank as my top five reasons for Technology Transfer failure:
1) Product / process not properly defined.
If the product is not well characterised and the production process not well defined then assumptions usually get made (and you know what they say about assumptions). This can result in “minor” or cosmetic issues (such as tablets the wrong colour being produced) to major quality deviations, batch rejects and 483’s for lack of process control being issued.
Although in general each process step is described in the process definition, there is often a failure to define critical process steps and critical parameters in short – the failure to define the process Design Space. This usually results I unacceptable delays resolving process parameter deviations, unwanted side effects and unanticipated impurities leading to the inability of the product to meet product specifications.
2) Lack of Experience
By this I am referring to the lack of personnel experience with the manufacture of similar products or type of product. This can lead to gross assumptions being made and an over reliance on process descriptions and SOPs, neither of which are usually detailed enough to allow inexperienced staff to carry out the process (be it the actual manufacturing or performing an analytical method) in the intended manner, or to be able to recognise and flag up anomalies, faults or irregularities in a timely manner if at all. Quite often this will result in out of specification products and significant deviation / CAPA generation activities being required.
3) Lack of a Technology Transfer Plan
There can be over 75 different processes which have to be performed during a typical technology transfer programme. These processes all have to be planned I properly in sequence and in concert with each other. There is often no pre-constructed Technology Transfer plan, and even if a plan exists, quite often there is a failure to assign specific responsibilities to the tasks within the plan. Making-it-up-as-you-go never works except for the very simplest transfers. Lack of a transfer plan usually leads to confusion, late delivery and often as not, the requirement to repeat tasks that have already been performed.
4) Use of substitute equipment
It’s very unusual for a receiving site to have exactly the same analytical and production equipment as the donor site, and even if it does, the chances are that one is a later version of the other.
This means that no two pieces of equipment will work exactly the same as each other which can lead to unintentional differences in both processing and testing the product. This is especially true for biologics production systems which can be very sensitive to even slight changes in process or testing conditions.
Sometimes I have even seen one piece of equipment substituted for another one that does the same (almost) as the first – such as membrane filtration units instead of depth filters, or impeller mixers instead of Z-blade mixers – resulting in amazement when the end products are not to the same specification. Of course scaling up of a process quite often falls foul of this type of thinking – a bigger piece of equipment although it looks the same and works in the same way often doesn’t perform in the same way as many people who substituted a larger bioreactor for a smaller one have found out to their cost.
5) Dedicated Technology Transfer Manager.
It always amazes me how often the task of managing a technology transfer project is given to someone who already has full time role on a secondary basis, on the grounds that it doesn’t need a dedicated manager or specialist. In all but the simplest transfers, it is a false economy to do this – one missed or mis-timed step can lead to significant time-delays and costs.
53,552 total views, 2 views today