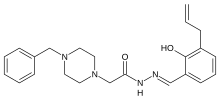
pac 1
Cancer drug tested in pet dogs is now bound for human trials
Medical Xpress
If PAC-1 (pack one) makes it through the U.S. Food and Drug Administration’s Investigational New Drug review, the first human (Phase I) clinical trial of the drug will begin in mid-2014. The investor, who wishes to … Procaspase-3 has long been an …
read all at
http://medicalxpress.com/news/2013-07-cancer-drug-pet-dogs-bound.html
www.ncbi.nlm.nih.gov/pmc/articles/PMC3113694/ this gives srtucture of pac-1
http://medicalxpress.com/partners/university-of-illinois-at-urbana-champaign/

A cell undergoing apoptosis. The dying cell blebs apart and sends signals to thephagocytes, which are part of the immune system, to engulf it.
PAC-1 (first procaspase activating compound) is a synthesized chemical compound that selectively induces apoptosis, or cell suicide, in cancerous cells. PAC-1 has shown good results in mouse models and is being further evaluated for use in humans. In 2010 a published study showed PAC-1 to be safe to research dogs, and a second study published later that same year reported that a PAC-1 derivative (called S-PAC-1) was well tolerated in a small Phase I Clinical Trial of pet dogs with lymphoma. Even at low doses of S-PAC-1, tumors regressed in 1/6 dogs, and the disease was stabilized (no additional tumor growth) in 3/6 dogs.
PAC-1 (pronounced “pack one”) was discovered in Paul Hergenrother’s labs at theUniversity of Illinois at Urbana-Champaign during a process that screened many chemicals for anti-tumor potential. This molecule, when delivered to cancer cells, signals the cells to self-destruct by activating an “executioner” protein, procaspase-3. Then, the activated executioner protein begins a cascade of events that destroys the machinery of the cell.
This cascade of events is named apoptosis. Apoptosis is self-induced in cells to combat infections or DNA damage. For instance, when a cell in one’s body is infected with a bacterium or virus, it will self-destruct to take away the resources needed by the virus to proliferate. Apoptosis is also found to help in embryo development (destroying the webbing in between an embryo’s fingers to separate the fingers) and the regular replenishment of cells that are constantly being used up or destroyed (cells that line the intestinal tract), also called homeostasis.

A cell undergoing apoptosis. The dying cell blebs apart and sends signals to thephagocytes, which are part of the immune system, to engulf it.
The problem lies when one part of the apoptosis pathway is broken. Normally, the balance between cell division and apoptosis is rigorously regulated to keep the integrity of organs and tissues. Examples of broken apoptosis pathways occur in many cancers. If old lung cells cannot self-destruct to make room for new lung cells, a large mass of cells form and a tumor is made.
In many cases, the apoptotic pathway is disrupted because procaspase-3, the executioner protein, cannot be activated by the cell. This is analogous to an executioner who does not have orders to kill. Without the orders, the condemned will not die. The same analogy can be made with procaspase-3. Without activated procaspase-3, the apoptotic cascade will not occur and the cell will not destroy itself no matter how necessary it may be. PAC-1 acts a replacement order that works and bypasses the lawyers, court orders, and governor’s calls. It will activate procaspase-3 indiscriminately.
How PAC-1 affects the apoptotic process
In cells, the executioner protein, caspase-3, is stored in its inactive form, procaspase-3. This way, the cell can quickly undergo apoptosis by activating the protein that is already there. This inactive form is called a zymogen. Procaspase-3 is known to be inhibited by low levels of zinc. PAC-1 activates procaspase-3 by chelating zinc, thus relieving the zinc-mediated inhibition. This allows procaspase-3 to be an active enzyme, and it can then cleave another molecule of procaspase-3 to active caspase-3. Caspase-3 can further activate other molecules of procaspase-3 in the cell, causing an exponential increase in caspase-3 concentration. PAC-1 facilitates this process and causes the cell to undergo apoptosis quickly.[1]
Unfortunately, a selectivity problem arises because procaspase-3 is present in most cells of the body. However, it has been shown that in many cancers, including certain neuroblastomas, lymphomas, leukemias, melanomas, and liver cancers, procaspase-3 is present in higher concentrations.[1] For instance, lung cancer cells can have over 1000 times more procaspase-3 than normal cells.[1] Therefore, by controlling the dosage, one can achieve selectivity between normal and cancerous cells.
Thus far, PAC-1 seems promising as a new anti-tumor drug. It is synthetically available and a few mouse trials have been performed with moderate success. PAC-1 is the first of many small molecules to directly influence the apoptotic machinery of cells.
References
- Putt KS, Chen GW, Pearson JM, Sandhorst JS, Hoagland MS, Kwon JT, Hwang SK, Jin H, Churchwell MI, Cho MH, Doerge DR, Helferich WG, Hergenrother PJ. (2006). “Small-molecule activation of procaspase-3 to caspase-3 as a personalized anticancer strategy. Nat. Chem. Biol”. Nature chemical biology 2 (10): 543–50. doi:10.1038/nchembio814. PMID 16936720.
- Peterson, Q. P.; Goode, D. R.; West, D. C.; Ramsey, K. N.; Lee, J. J.; Hergenrother, P. J. “PAC-1 Activates Procaspase-3 in vitro Through Relief of Zinc-Mediated Inhibition” J. Mol. Biol. 2009, 388, 144-158.
- Peterson, Q. P.; Hsu, D. C.; Goode, D. R.; Novotny, C. J.; Totten, R. K. Hergenrother, P. J.; “Procaspase-3 Activation as an Anti-Cancer Strategy: Structure-Activity Relationship of PAC-1, and its Cellular Co-Localization with Caspase-3” J. Med. Chem. 2009, 52, 5721-5731.
- Lucas, P. W.; Schmit, J. M.; Peterson, Q. P.; West, D. C.; Hsu, D. C.; Novotny, C. J.; Dirikoul, L.; Deorge, D. R.; Garrett, L. D.; Hergenrother, P. J., Fan, T. M. “Pharmacokinetics and Derivation of an Anticancer Dosing Regimen for PAC-1, a Preferential Small Molecule Activator of Procaspase-3, in Healthy Dogs” Invest. New Drugs. 2010, in press published on web May 25, 2010.
Peterson, Q. P.; Hsu, D. C.; Novotny, C. J.; West, D. C.; Kim, D.; Schmit, J. M.; Dirikolu, L.; Hergenrother, P. J.; Fan, T. M. “Discovery and Canine Preclinical Assessment of a Nontoxic Procaspase-3-Activating Compound” Cancer Res. 2010, 70, 7232-7241













Sorry, the comment form is closed at this time.