Special drugs
Chinese Plant Compound , Triptolide Wipes out Cancer in 40 Days, Say new Research.
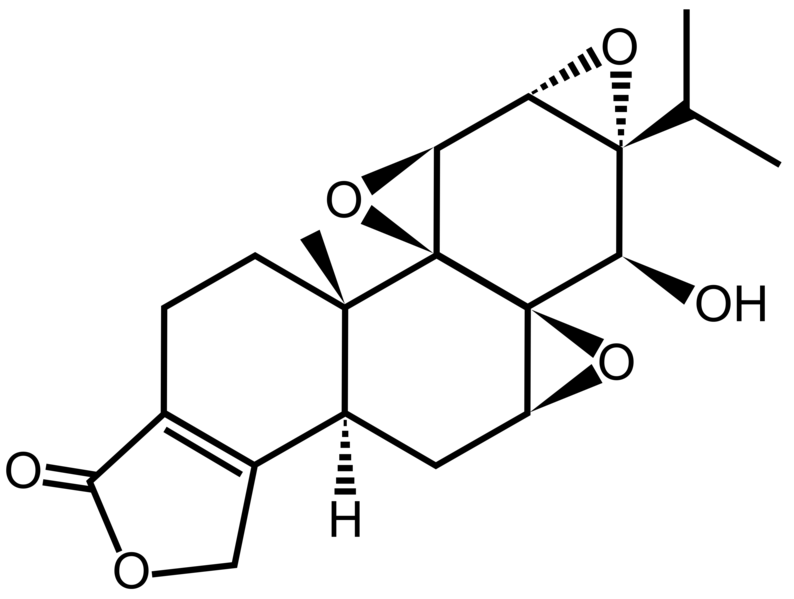
Triptolide is a diterpenoid epoxide found in the Thunder God Vine, Tripterygium wilfordii. It has in vitro and in vivo activities against mouse models of polycystic kidney disease[1] and pancreatic cancer, but its physical properties limit its therapeutic potential.[2] Consequently, a synthetic prodrug, minnelide, is being studied clinically instead.[2]
A little-known plant with a truly bizarre name is now making headlines as a cancer killer, with the compound of the plant vanishing tumors in mice with pancreatic cancer. Known as the ‘thunder god vine’ or lei gong teng, the Chinese plant is actually integrated into Chinese medicine and has been used for ages in remedying a number of conditions including rheumatoid arthritis.
According to the new research out of the University of Minnesota’s Masonic Cancer Center, the thunder god plant compound led to no signs of tumors after a 40 day period — even after discontinuing the treatment. Published in the journal Science Translational Medicine
http://stm.sciencemag.org/content/4/156/156ps21.full?sid=5203733d-ae1d-438e-9d36-b8bd9f16cd8d
Pancreas Cancer Meets the Thunder God
- Sunil R. Hingorani and
- John D. Potter
Sci Transl Med 17 October 2012 4:156ps21. DOI:10.1126/scitranslmed.3004956
- Rohit Chugh,et al
Sci Transl Med 17 October 2012 4:156ra139. DOI:10.1126/scitranslmed.3004334
and funded by the National Institutes of Health, even the scientists working on the project were stunned by the anti-cancer properties of the compound. Known to contain something known as triptolide, which has been identified as a cancer fighter in previous research, it is thought to be the key component that may be responsible for the anti-tumor capabilities.

And just like with numerous other powerful substances like turmeric and ginger, mainstream science is still slowly confirming what many traditional practitioners have known for their entire lives. This is, of course, due to the fact that there is simply no moneyfor major corporations in researching the healing powers of natural herbs and compounds such as the compound found in the thunder god vine. Turmeric and ginger, for example, have been found to be amazing anti-cancer substances that are virtually free compared to expensive and dangerous cancer drugs.
References
- Leuenroth, Stephanie (2007). “Triptolide is a traditional Chinese medicine-derived inhibitor of polycystic kidney disease”. PNAS104 (11): 4389-4394. doi:10.1073/pnas.0700499104. Retrieved 18 October 2012.
- Chugh, Rohit (2012). “A Preclinical Evaluation of Minnelide as a Therapeutic Agent Against Pancreatic Cancer”. Science Translational Medicine4 (156): 156ra139. doi:10.1126/scitranslmed.3004334. Retrieved 18 October 2012.


 Monoclonal antibody
Monoclonal antibody
Genzyme’s LEMTRADA™ (alemtuzumab) Application for MS Accepted for Review by the FDA
January 28, 2013
Genzyme, a Sanofi Company (EURONEXT: SAN and NYSE: SNY), announced that the U.S. Food and Drug Administration (FDA) has accepted for review the company’s supplemental Biologics License Application (sBLA) file seeking approval of LEMTRADA (alemtuzumab) for the treatment of relapsing multiple sclerosis (RMS). The company also reported key highlights from the U.S. launch of once-daily, oral AUBAGIO (teriflunomide).
LEMTRADA sBLA Accepted by FDA
The FDA has accepted for standard review the company’s sBLA file seeking approval of LEMTRADA. Genzyme expects FDA action on the application in the second half of 2013. Genzyme has already submitted its marketing authorization application for LEMTRADA to the European Medicines Agency (EMA) and the review process is underway. The Committee for Medicinal Products for Human Use (CHMP) opinion for LEMTRADA is expected in Q2 2013.
The LEMTRADA clinical development program includes CARE-MS I and CARE-MS II (Comparison of Alemtuzumab and Rebif® Efficacy in Multiple Sclerosis), randomized Phase III studies comparing LEMTRADA to a standard of care MS treatment, Rebif,in patients with relapsing-remitting MS who were naïve to prior treatment or who had relapsed while on prior therapy, respectively. Genzyme announced publication of results of these studies in The Lancet in November 2012.
AUBAGIO Early Launch Indicators in the U.S.
Since its launch in October, once-daily, oral AUBAGIO has shown very encouraging early launch indicators among U.S. prescribers.1 Key highlights from the launch include:
- More than 80 percent of MS specialists in the U.S. have prescribed AUBAGIO;
- Approximately 1 in 5 patients prescribed AUBAGIO were treatment-naïve;
- More than 50 percent of AUBAGIO patients were most recently on Copaxone® or Avonex®
“Genzyme is making a difference for people living with MS and realizing its vision of being leaders in MS,” said Genzyme President and CEO, David Meeker, M.D. “The initial uptake of AUBAGIO by U.S. prescribers shows the importance of a once-daily oral option in MS. In addition, the acceptance of the LEMTRADA file in the U.S. marks another important milestone in bringing this potentially transformative therapy to MS patients. We look forward to a series of product launches in 2013 in Europe and other major markets.”
AUBAGIO is approved for use in both the U.S. and Australia.
About Alemtuzumab/LEMTRADA™
Alemtuzumab is a monoclonal antibody that selectively targets CD52, a protein abundant on T and B cells. Treatment with alemtuzumab results in the depletion of circulating T and B cells thought to be responsible for the damaging inflammatory process in MS. Alemtuzumab has minimal impact on other immune cells. The acute anti-inflammatory effect of alemtuzumab is immediately followed by the onset of a distinctive pattern of T and B cell repopulation that continues over time, rebalancing the immune system in a way that potentially reduces MS disease activity.
Alemtuzumab (marketed as Campath, MabCampath or Campath-1H and currently under further development as Lemtrada) is a monoclonal antibody used in the treatment of chronic lymphocytic leukemia (CLL), cutaneous T-cell lymphoma (CTCL) and T-cell lymphoma. It is also used in some conditioning regimens for bone marrow transplantation, kidney transplantation and Islet cell transplantation.
Alemtuzumab binds to CD52, a protein present on the surface of mature lymphocytes, but not on the stem cells from which these lymphocytes are derived. After treatment with alemtuzumab, these CD52-bearing lymphocytes are targeted for destruction.
Alemtuzumab is used as second-line therapy for CLL. It was approved by the US Food and Drug Administration for CLL patients who have been treated with alkylating agents and who have failed fludarabine therapy. It has been approved by Health Canada for the same indication, and additionally for CLL patients who have not had any previous therapies.
It is also used under clinical trial protocols for treatment of some autoimmune diseases, such as multiple sclerosis, in which it shows promise.[1][2] Alemtuzumab was withdrawn from the markets in the US and Europe in 2012 to prepare for a higher-priced relaunch aimed at multiple sclerosis.[3]
A complication of therapy with alemtuzumab is that it significantly increases the risk for opportunistic infections, in particular, reactivation of cytomegalovirus.
- Drug may reverse MS brain damage”. 22 Oct 2008.
- “Sanofi and Genzyme Report New Positive Data from First Phase III Study with MS Drug”. 24 Oct 2011.
- “Sanofi withdraws Campath in US and EU”. Pharma Times Online. August 21, 2012.
DR ANTHONY CRASTO, PhD, ICT Organic chemistry, Currently working with GLENMARK GENERICS LTD research centre as Principal Scientist, process research (bulk actives) at Mahape, Navi Mumbai, India, helping millions, million hits on google on all organic chemistry websites, Hands on experience in developing novel routes for drug molecules and implementation on commercial scale. several international patents published.pushing boundaries, one lakh connections on all networking sites



(Z)-5-Tetradecen-1-ol, mw 212.37, formula C14H20O CAS 40642-42-0
Odorant receptors, present on nasal sensory neurons, perceive volatile compounds and regulate animal behavior such as reproduction. The nature of the ligands interacting with these receptors is, however, largely unknown.
Keiichi Yoshikawa and colleagues, University of Tokyo, Japan, shed new light on this issue. The researchers demonstrated that, in mice, preputial gland cells generate and secrete into the urine the unsaturated aliphatic alcohol (Z)-5-tetradecen-1-ol (pictured). This compound is regulated by the male hormone testosterone and acts as a natural agonist of the mouse odorant receptor Olfr288, affecting attractiveness to female mice. The urine of males lacking (Z)-5-tetradecen-1-ol, in fact, failed to attract females.

By identifying a novel receptor-ligand interaction in the mouse olfactory system, this study offers new insights into the complex chemistry regulating reproductive behavior.
- An unsaturated aliphatic alcohol as a natural ligand for a mouse odorant receptor,
K. Yoshikawa, H. Nakagawa, N. Mori, H. Watanabe, K. Touhara,
Nature Chem. Biol. 2013.
DOI: 10.1038/nchembio.1164 - Ohloff, G. et al. 1977. Helv. Chim. Acta. 60:1161-1174.
- http://www.cas-msds.com/40642-42-0
-
Bestmann, H.J., Brosche, T., Koschatzky, K.H., Michaelis, K., Platz, H., Vostrowsky, O., and Knauf, W. 1980. Pheromone XXX. Identifizierung eines neuartigen pheromonkomplexes aus der graseule Scotia exclamationis. Tetrahedron Lett. 21:747-750. Kelkar, S.V., Reddy, G.B., and Kulkarni, G.H. 1989. Indian J. Chem. Sect. B. 28:980-981. Ohloff, G., Vial, C., Näf, F., and Pawlak, M. 1977. Stereoselective syntheses of the isomeric 5, 10-pentadecadienals. Helv. Chim. Acta. 60:1161-1174.  DR ANTHONY MELVIN CRASTO Ph.D
DR ANTHONY MELVIN CRASTO Ph.D amcrasto@gmail.com
MOBILE-+91 9323115463GLENMARK SCIENTIST , NAVIMUMBAI, INDIA
amcrasto@gmail.com
MOBILE-+91 9323115463GLENMARK SCIENTIST , NAVIMUMBAI, INDIADR ANTHONY CRASTO, PhD, ICT Organic chemistry, Currently working with GLENMARK GENERICS LTD research centre as Principal Scientist, process research (bulk actives) at Mahape, Navi Mumbai, India, helping millions, million hits on google on all organic chemistry websites, Hands on experience in developing novel routes for drug molecules and implementation on commercial scale. several international patents published.pushing boundaries, one lakh connections on all networking sites


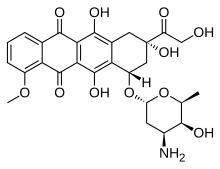 |
|
|---|---|
| (7S,9S)-7-[(2R,4S,5S,6S)-4-amino-5-hydroxy-6-methyloxan-2-yl]oxy-6,9,11-trihydroxy-9-(2-hydroxyacetyl)-4-methoxy-8,10-dihydro-7H-tetracene-5,12-dione |
LINK
Heat–activated liposome encapsulated doxorubicin – NIHR Horizon …Heat–activated liposome encapsulated doxorubicin (ThermoDox) for hepatocellular carcinoma.
Zhejiang Hisun Pharma inlicenses China rights to liver cancer drug (ThermoDox) from Celsion Corporation
About ThermoDox
The study is designed to evaluate the efficacy of ThermoDox® in combination with RFA when compared to patients who receive RFA alone as the control. The primary endpoint for the study is progression-free survival (PFS) with a secondary confirmatory endpoint of overall survival. ThermoDox® has been granted orphan drug designation in both the U.S. and Europe. In addition to meeting the U.S. FDA and European EMA enrollment objectives, the Phase 3 Study has also enrolled a sufficient number of patients to support registration filings in China, South Korea and Taiwan, three of the largest potential markets for ThermoDox® around the world.
Terms of the Deal
Under the terms of the agreement, Hisun will pay $5 million to Celsion immediately, while Celsion will provide Hisun with support for its ThermoDox® manufacturing development program. This payment is non-refundable and comes in advance of Celsion’s expected reporting of results from its pivotal Phase III trial (the HEAT Study) in hepatocellular carcinoma (HCC), also known as primary liver cancer later this month.
In addition, the companies anticipate signing an agreement in which Celsion provides Hisun an exclusive option to license ThermoDox® for the Greater China market, which includesChina, Hong Kong and Macau. This option period will be secured by a second $5 millionpayment that must be received by Celsion from Hisun within 60 days after execution of the Technology Development Agreement. The key provisions of the anticipated license agreement have been negotiated and agreed to by the parties and provide a basis for a definitive contract. These provisions are:
- A credit of $10 million from the two payments ($5 million for the technology development agreement and $5 million for the exclusive option) toward a non-refundable upfront license payment of $25 million due to Celsion at signing of the definitive license agreement.
- An approximate 10 year total value to Celsion of well over several hundred million US dollars, which includes:
- $55 million in upfront milestone and regulatory milestone payments within the next 18 months;
- $45 million in milestone payments for reaching certain sales targets; and
- Escalating double-digit royalties on net sales of ThermoDox® in the Greater China Territory.
- Hisun will serve as both the manufacturer and distributor of the ThermoDox® drug product for the Greater China Territory, and also take responsibility for local regulatory activities including submitting approvals in China to the state Food and Drug Administration (sFDA).
Deal Rationale
“Pursuing this arrangement with Hisun allows us to evaluate the fastest path to the Chinamarket, potentially the largest opportunity in the world for ThermoDox®. A long-term partnership will provide the greatest synergies with respect to sales, marketing, distribution, and manufacturing, which could ensure significant value to the ThermoDox® asset,” saidMichael H. Tardugno, Celsion’s President and Chief Executive Officer. “In addition, this partnership provides Hisun and Celsion with immediate access to an accelerated pathway for sFDA review and approval of ThermoDox®, a business strategy with exceptional potential to serve China’s HCC population, and strong, uncompromised economics for both parties.”
Mr. Hua Bai, CEO and Chairman of Hisun, stated, “We are extremely excited to pursue this arrangement with Celsion. Hisun is well positioned to provide ThermoDox® — potentially one of the most important and innovative drugs to treat HCC to patients in China, the world’s largest market. China is one of the countries with the highest HCC incidence and mortality and, up until now, there has not been any standard of care for treating HCC in China.
This joint effort will most likely facilitate the local manufacturing and commercial launch inChina, thereby providing physicians with more options for better care and prolonging the survival of HCC patients. In the meantime, we are also hopeful that this collaboration will enable Hisun to increase its focus on more innovative drugs. Given the fact that we are a leading Chinese pharmaceutical company with international standards of R&D and manufacturing technology, Hisun will seek to manufacture and supply the global markets, along with distribution exclusivity in Greater China. This venture will help spearhead Hisun’s globalization in manufacturing and commercialization capabilities.”
About Primary Liver Cancer
Primary liver cancer is one of the most deadly forms of cancer and ranks as the fifth most common solid tumor cancer. The incidence of primary liver cancer today is approximately 26,000 cases per year in the United States, approximately 40,000 cases per year in Europeand is rapidly growing worldwide at approximately 750,000 cases per year, 55 percent of which are in China, due to the high prevalence of Hepatitis B and C in developing countries. . The World Health Organization estimates that primary liver cancer may become the number one cancer worldwide, surpassing lung cancer, by 2020.
The standard first-line treatment for liver cancer is surgical resection of the tumor; however, 90% of patients are ineligible for surgery. Radio frequency ablation (RFA) has increasingly become the standard of care for non-resectable liver tumors, but the treatment becomes less effective for larger tumors. There are few non-surgical therapeutic treatment options available as radiation therapy and chemotherapy are largely ineffective in the treatment of primary liver cancer.
Doxorubicin trade name Adriamycin; also known ashydroxydaunorubicin) is a drug used in cancer chemotherapy. It is an anthracyclineantibiotic, closely related to the natural product daunomycin, and like all anthracyclines, it works by intercalating DNA, with the most serious adverse effect being life-threatening heart damage. It is commonly used in the treatment of a wide range of cancers, includinghematological malignancies, many types of carcinoma, and soft tissue sarcomas.
The drug is administered intravenously, as the hydrochloride salt. It may be sold under the brand names Adriamycin PFS, Adriamycin RDF, or Rubex.[2] Doxorubicin is photosensitive, and containers are often covered by an aluminum bag and/or brown wax paper to prevent light from affecting it.
Doxorubicin is available in liposome-encapsulated forms as Doxil, Caelyx and Myocet.
Doxorubicin is commonly used to treat some leukemias and Hodgkin’s lymphoma, as well as cancers of the bladder, breast, stomach,lung, ovaries, thyroid, soft tissue sarcoma, multiple myeloma, and others.[2] Commonly used doxorubicin-containing regimens are AC (Adriamycin, cyclophosphamide), TAC (Taxotere, CA), ABVD (Adriamycin, bleomycin, vinblastine, dacarbazine), BEACOPP, CHOP(cyclophosphamide, Adriamycin, vincristine, prednisone) and FAC (5-fluorouracil, Adriamycin, cyclophosphamide).
Doxil (see below) is used primarily for the treatment of ovarian cancer where the disease has progressed or recurred after platinum-based chemotherapy, or for the treatment of AIDS-related Kaposi’s sarcoma.[7]
- Laginha, K.M. “Determination of Doxorubicin Levels in Whole Tumor and Tumor Nuclei in Murine Breast Cancer Tumors.”Clinical Cancer Research. October 1, 2005. Vol. 11 (19). Retrieved on April 19, 2007.
- “Doxorubicin (Systemic).” Mayo Clinic. Last updated on: June 15, 1999. Retrieved on April 19, 2007. Archived April 3, 2007 at the Wayback Machine
- Weiss RB (December 1992). “The anthracyclines: will we ever find a better doxorubicin?”. Seminars in Oncology 19 (6): 670–86. PMID 1462166.
- Tan C, Tasaka H, Yu KP, Murphy ML, Karnofsky DA (March 1967). “Daunomycin, an antitumor antibiotic, in the treatment of neoplastic disease. Clinical evaluation with special reference to childhood leukemia”. Cancer 20 (3): 333–53. doi:10.1002/1097-0142(1967)20:3<333::AID-CNCR2820200302>3.0.CO;2-K.PMID 4290058.
- Arcamone F, Cassinelli G, Fantini G, et al. (1969). “Adriamycin, 14-hydroxydaunomycin, a new antitumor antibiotic from S. peucetius var. caesius“. Biotechnol Bioeng 11 (6): 1101–10. doi:10.1002/bit.260110607. PMID 5365804.
- Di Marco A, Gaetani M, Scarpinato B (February 1969). “Adriamycin (NSC-123,127): a new antibiotic with antitumor activity”. Cancer Chemother Rep 53 (1): 33–7. PMID 5772652.
- “DOXIL Product Information.” Ortho Biotech Products, L.P. Retrieved on April 19, 2007. Archived September 21, 2007 at the Wayback Machine


ABRAXANE, a microtubule inhibitor, is an albumin-bound form of paclitaxel with a mean particle size of approximately 130 nanometers. Paclitaxel exists in the particles in a non-crystalline, amorphous state. ABRAXANE is supplied as a white to yellow, sterile, lyophilized powder for reconstitution with 20 mL of 0.9% Sodium Chloride Injection, USP prior to intravenous infusion. Each single-use vial contains 100 mg of paclitaxel (bound to human albumin) and approximately 900 mg of human albumin (containing sodium caprylate and sodium acetyltryptophanate). Each milliliter (mL) of reconstituted suspension contains 5 mg paclitaxel. ABRAXANE is free of solvents.
The active agent in ABRAXANE is paclitaxel.
The chemical name for paclitaxel is 5β,20-Epoxy-1,2α,4,7β,10β,13α-hexahydroxytax-11-en-9-one 4,10-diacetate 2-benzoate 13-ester with (2R,3S)-N-benzoyl-3-phenylisoserine.
Paclitaxel is a white to off-white crystalline powder with the empirical formula C47H51NO14 and a molecular weight of 853.91. It is highly lipophilic, insoluble in water, and melts at approximately 216°C to 217°C.
A drug that Celgene acquired in its $2.9 billion acquisition of abraxis bioscience in 2010 is showing promise against the notoriously hard-to-treat pancreatic cancer.
The disease kills 38,000 Americans each year, nearly as many as those who die from breast cancer and a much larger percentage rate of those diagnosed. So observers see promise from the results of a new study that showed Celgene’s Abraxane plus gemcitabine, the standard drug for pancreatic cancer, led to a median survival rate of 8.5 months, compared with 6.7 months for those who received gemcitabine alone. It also improved survival rates after one and two years, the company reported today.
The results were pretty much in line with what investors were expecting, according to The New York Times. Analysts saw potential when Celgene added Abraxane to its portfolio because it is a “platform drug” used to treat several forms of cancer. It was approved in the U.S. in October for treating lung cancer and is approved in the U.S. and many other countries for treating breast cancer.
The drug had sales of $320 million in the first 9 months of last year. The global market for pancreatic cancer treatments is only about $700 million, according to industry research firm Decision Resources. The firm estimates the U.S. market will grow to $829 million in the U.S. by 2019. Celgene said it would apply for approval in the the first half of the year for Abraxane the new indication, according to The New York Times.
But Abraxane is not the only investigational drug showing promise for treating pancreatic cancer. As the newspaper points out, a trial of Folfirinox, a combination of four generic cancer drugs showed a median survival of 11.1 months compared with 6.8 months for those getting gemcitabine. On the other hand, patients have to wear an infusion pump to take Folfirinox and it can be hard to tolerate.
“The simplicity of Abraxane plus gemcitabine may be attractive to physicians and patients,” Dr. Neal J. Meropol of the University Hospitals and Case Western Reserve University in Cleveland tells The New York Times.

AT13148
AT13148 is a multi-AGC kinase, ATP-competitive inhibitor, identified utilizing high-throughput X-ray crystallography and fragment-based lead discovery techniques. AT13148 caused substantial blockade of AKT, p70S6K, PKA, ROCK and SGK substrate phosphorylation and induction of apoptosis in both a concentration and time-dependent manner in cancer cells with clinically relevant genetic defects both in vitro and in vivo.
Date: January 17, 2013
Cancer Research UK and its commercial arm Cancer Research Technology (CRT) are launching a trial of an experimental drug shown to simultaneously block many enzymes that control cancer cell growth and death. The ‘master-switch’ experimental drug, owned by Astex Pharmaceuticals, could potentially treat a range of cancer types.
Cancer Research UK’s Drug Development Office (DDO) will fund, manage and sponsor this early-stage Phase 1 clinical trial of up to 40 patients at The Institute of Cancer Research, London, and The Royal Marsden Hospital.
The drug, AT13148, is one of eight drugs to be developed through Cancer Research UK’s Clinical Development Partnerships (CDP) program, which is a joint initiative between the charity’s DDO and CRT. The program develops promising cancer drugs that pharmaceutical companies do not have the resources to progress through early phase clinical trials to see if they can benefit cancer patients. Without this program many promising drugs would be left on the shelves gathering dust.
AT13148 is a type of drug called a kinase inhibitor. Research in the laboratory has shown it can simultaneously block several different enzymes that control cell growth and cell death, and the drug killed a range of cancer cell types including sarcoma, breast and prostate. Many drugs block only a single enzyme and scientists hope that switching off cell signals at multiple points at the same time could increase the effectiveness of this drug.
Dr Udai Banerji, Cancer Research UK senior clinical lecturer at The Institute of Cancer Research and honorary consultant in medical oncology at The Royal Marsden, said: “There’s an urgent need to discover and develop new ways to beat cancers that do not respond to the treatments we have at the moment. By targeting cancer cells at a range of weak spots instead of just one, tumors will be less likely to develop resistance to this treatment. We’re very pleased that this multi-target drug has now reached patients in the clinical trial stage.
“It’s thanks to the generosity and time of patients that it’s possible to carry out clinical trials like this that could lead to new treatments for patients in the future.”
The novel agent came into the DDO requiring extensive preclinical development work.
Cancer Research UK scientists at the DDO and those funded at The Institute of Cancer Research demonstrated that the drug would be suitable for patients and, working together with specialist manufacturing organisations, developed a sophisticated method to make the drug in its most basic form. Finally, Cancer Research UK’s Formulation Unit at the University of Strathclyde manufactured the drug for the trial.
The molecule was originally discovered by scientists on the PKB drug discovery program, a collaboration between Astex Pharmaceuticals, CRT and The Institute of Cancer Research, which ran from 2003 through to 2006.
Harren Jhoti, Astex Pharmaceuticals president and director, said: “We are very gratified with the progress that the collaboration has achieved and that this work has progressed into the clinic.”
Astex Pharmaceuticals can decide to develop the drug further based on the Phase 1/2a clinical trial data. If it chooses not to, Cancer Research Technology has the rights to secure an alternative partner and ensure the drug has every possible chance of reaching patients. The charity will receive a share of revenues generated by the drug to be channelled back into life-saving research.
Dr. Victoria John, head of clinical partnerships at Cancer Research UK’s Drug Development Office, said: “We’re delighted to open the first clinical trial of this experimental drug to find out if it can benefit cancer patients in the future.
“This molecule was brought to us at a very early stage in its development and, with the preclinical work now completed, we’re extremely pleased it’s obtained regulatory approval to enter the clinic.
“We’ve developed this molecule through our Clinical Development Partnerships initiative that has allowed us to form strong links with industry to take this promising drug forward. Without the program it may have remained undeveloped and the clinical trial simply would not have been possible.”
CAS: 1056901-62-2
Synonym: AT13148; AT 13148; AT-13148.
IUPAC/Chemical name:
(S)-1-(4-(1H-pyrazol-4-yl)phenyl)-2-amino-1-(4-chlorophenyl)ethanol
Chemical Formula: C17H16ClN3O
Molecular Weight: 313.78
Elemental Analysis: C, 65.07; H, 5.14; Cl, 11.30; N, 13.39; O, 5.10
AT13148 is an orally active small molecule inhibitor of PKB/Akt and p70S6 kinase, key enzymes in the PI3K/PKB/mTOR tumor cell survival pathway. AT13148 has the potential to be a very effective inhibitor of AKT dependent tumors. In September 2008 Astex announced a partnership with Cancer Research UK and CRT to take AT13148 into development under the charity’s Clinical Development Partnerships (CDP) program. Under the terms of this agreement, Cancer Research UK’s Drug Development Office has carried out further development work on the agent, some of which is done in collaboration with the ICR
ref http://clincancerres.aacrjournals.org/content/early/2012/06/26/1078-0432.CCR-11-3313.abstract

36,627 total views, 2 views today















