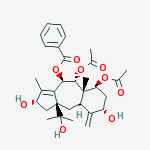
IUPAC Name: [(2S,4R,5R,5aS,6S,8S,9aR,10aS)-5,6-diacetyloxy-2,8-dihydroxy-10a-(2-hydroxypropan-2-yl)-3,5a-dimethyl-9-methylidene-2,4,5,6,7,8,9a,10-octahydro-1H-benzo[g]azulen-4-yl] benzoate | CAS Registry Number: 134955-83-2
Synonyms: Brevifoliol, CHEBI:555808, CID178222, 134955-83-2
Brevifoliol was first isolated from the leaves of the plant Taxus brevifolia (F. Balza et al Phytochemistry 30, p. 1613-1614 (1991)). The process of its isolation involved extracting the fresh leaves of Taxus wallichiana with ethyl alcohol to get an extract. The crude extract after concentration was diluted with water and partitioned between hexane, chloroform and ethyl acetate sequentially. The chloroform extract upon concentration yielded a dark brown residue. The resultant residue was subjected to column chromatography over silica gel and eluted with chloroform and chloroform-methanol gradient. Six fractions were collected and brevifoliol was isolated from fraction five by rechromatography over silica gel and eluting with hexane-ethyl acetate gradient.
Brevifoliol has been isolated from other species of Taxus including the Himalayan yew Taxus wallichiana which is available in India., Recently, the structure of brevifoliol has been revised and it was shown to belong to 11 (15→1) abeo taxoid bicyclic skeleton of formula (1). The isolation of brevifoliol from leaves of the plantTaxus wallichiana is also reported in S. K. Chattopadhyay et al Indian J. Chemistry 35B, 175-177(1996) as part of studies on the isolation of anticancer compounds. The process of this disclosure involved extracting the dried and crushed needles of Taxus wallichiana with methanol for 72 hours and the extract was concentrated in vacuo. The concentrate was diluted with water and extracted with hexane and chloroform respectively. Concentration of the chloroform phase under vacuum left a residue which was separated by column chromatography over silica gel. Fraction eluted with chloroform-methanol (98:5) contained brevifoliol which was further purified by re-chromatography over silica gel and eluted with chloroform-methanol (99:2). Fractions containing brevifoliol were combined and concentrated and recrystallized from pet-ether and ethyl acetate mixture to get brevifoliol as needles. In in vitro testing of brevifoliol, it was found to have significant anticancer activity against different cancer cell lines. The detection of anticancer activity in brevifoliol prompted the present investigators to develop an efficient processing technology for isolation of the compound in large quantities from the needles of the plant for further biological testing.
The prior art process of isolation of brevifoliol suffers from a number of disadvantages including partitioning of the aqueous extract with hexane and chloroform and repeated column chromatography to get the compound. Although the partitioning of the aqueous phase with organic solvents works on small scale, it forms thick emulsions on large scale partitioning process and creates hindrance in getting the fractions separated. Also, the use of repeated chromatography might be useful on small-scale isolation of brevifoliol, it is only cumbersome, tedious and not economical on large-scale process.
Felipe Balza, et al., “Brevifoliol, A Taxane From Taxus Brevifolla“, Phytochemistry, Pergamon Press, GB, vol. 30, No. 5, 1991, pp. 1613-1614
Patent No. U.S. 7,579,491

- (i) Dry and pulverize the leaves of the plant.
- (ii) Extract the dried leaves with an alcohol such as MeOH or EtOH at 20−40 °C over 3 days.
- (iii) Concentrate the alcoholic solution and adsorb the extract onto Celite.
- (iv) Dry the Celite adsorbate at 20−50 °C for up to 48 h.
- (v) Extract the dried adsorbate with 60−80 petroleum ether then CHCl3 and concentrate the CHCl3 extract.
- (vi) Subject the concentrated mixture to gross fractionation over a column of silica gel using CHCl3 add 2% MeOH in CHCl3.
- (vii) Subject the later eluate to further chromatography over alumina in petroleum ether using 10% EtOAc in petroleum ether.
- (viii) Recrystallise from EtOAc/petroleum ether as needles.
Advantages
One Response to “Process for Preparing Brevifoliol, US 7579491, CSIR, INDIA”
Sorry, the comment form is closed at this time.













very informative!