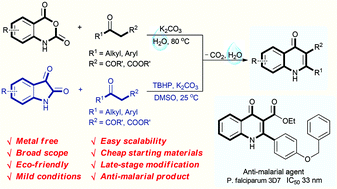
Abstract
An environmentally benign decarboxylative cyclization in water has been developed to synthesize 4-quinolones from readily available isatoic anhydrides and 1,3-dicarbonyl compounds. Isatins are also compatible for the reaction to generate 4-quinolones in the presence of TBHP in DMSO. This protocol provides excellent yields under mild conditions for a broad scope of 4-quinolones, and has good functional group tolerance. Only un-harmful carbon dioxide and water are released in this procedure. Moreover, the newly synthesized products have also been selected for anti-malarial examination against the chloroquine drug-sensitive Plasmodium falciparum 3D7 strain. 3u is found to display excellent anti-malarial activity with an IC50 value of 33 nM.
Eco-friendly decarboxylative cyclization in water: practical access to the anti-malarial 4-quinolones
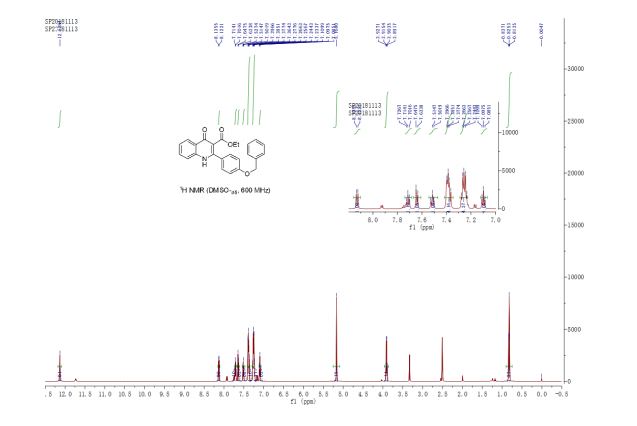
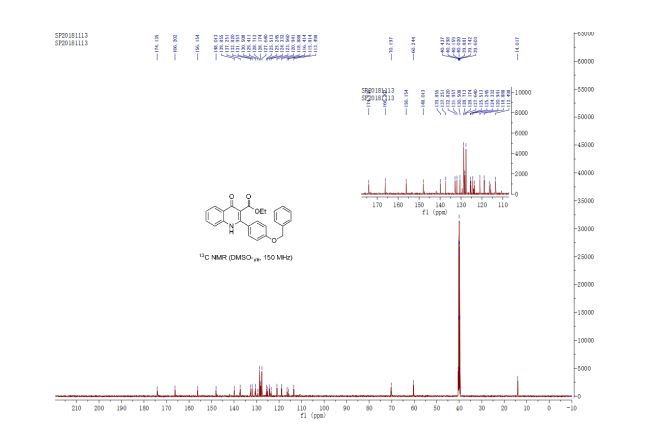
ethyl 2-(4-(benzyloxy)phenyl)-4-oxo-1,4-dihydroquinoline-3-carboxylate (3u) White solid, m.p. 288-289 oC;
1H NMR (600 MHz, DMSO-d6) δ 12.14 (s, 1H), 8.13 (d, J = 8.0 Hz, 1H), 7.72 (ddd, J = 8.4, 7.1, 1.5 Hz, 1H), 7.64 (d, J = 8.3 Hz, 1H), 7.52 (td, J = 8.5, 1.7 Hz, 1H), 7.43 – 7.35 (m, 4H), 7.29 – 7.21 (m, 4H), 7.10 (td, J = 7.5, 0.5 Hz, 1H), 5.17 (s, 2H), 3.91 (q, J = 7.1 Hz, 2H), 2.00 (s, 1H), 0.83 (t, J = 7.1 Hz, 3H) ppm;
13C NMR (150 MHz, DMSO-d6) δ 174.1, 166.2, 156.2, 148.0, 139.8, 137.2, 132.8, 132.0, 130.5, 129.4, 128.7, 128.2, 127.6, 125.5, 125.2, 124.3, 123.6, 120.9, 118.9, 116.4, 115.8, 113.5, 70.2, 60.2, 14.0 ppm;
HRMS (ESI) calcd for [C25H21NO4+H]+ 400.1471, found 400.1463.













Sorry, the comment form is closed at this time.