DOI: 10.1039/C8GC00078F, Tutorial Review
Recent advances in aqueous fluoroalkylation using various fluoroalkylation reagents are summarized in this review.
Fluoroalkylation reactions in aqueous media: a review
Abstract
This review highlights the progress of aqueous fluoroalkylation over the past few decades. Fluorine-containing functionalities are important design elements in new pharmaceuticals, agrochemicals, and functional materials, due to their unique effects on the physical, chemical, and/or biological properties of a molecule. Because the environmental concerns are receiving increasing attention in organic synthesis, the development of methods for the mild, environment-friendly, and efficient incorporation of fluorinated or fluoroalkylated groups into the target molecules is of broad interest. At the early stage, most of the fluoroalkylation reactions and their variants were thought in principle to be hydrophobic. Recently, the environment-benign fluoroalkylation reactions by taming nucleophilic, radical, or electrophilic fluoroalkylation reagents in water or in the presence of water have been explored, building a new prospect for green chemistry. The use of significant catalytic systems and/or the newly developed reagents is the key to the success of these reactions. Water is used as a (co)solvent and/or a reactant in aqueous fluoroalkylation, including trifluoromethylation, difluoromethylation, monofluoromethylation, trifluoroethylation, perfluoroalkylation, trifluoromethylthiolation, and other conversions, under environment-friendly conditions. Although great accomplishments have been achieved, they are just the tip of the iceberg with a wide scope for improvement. This review will draw great attention and inspire more contributions in the development of new aqueous fluoroalkylation reactions
Conclusion
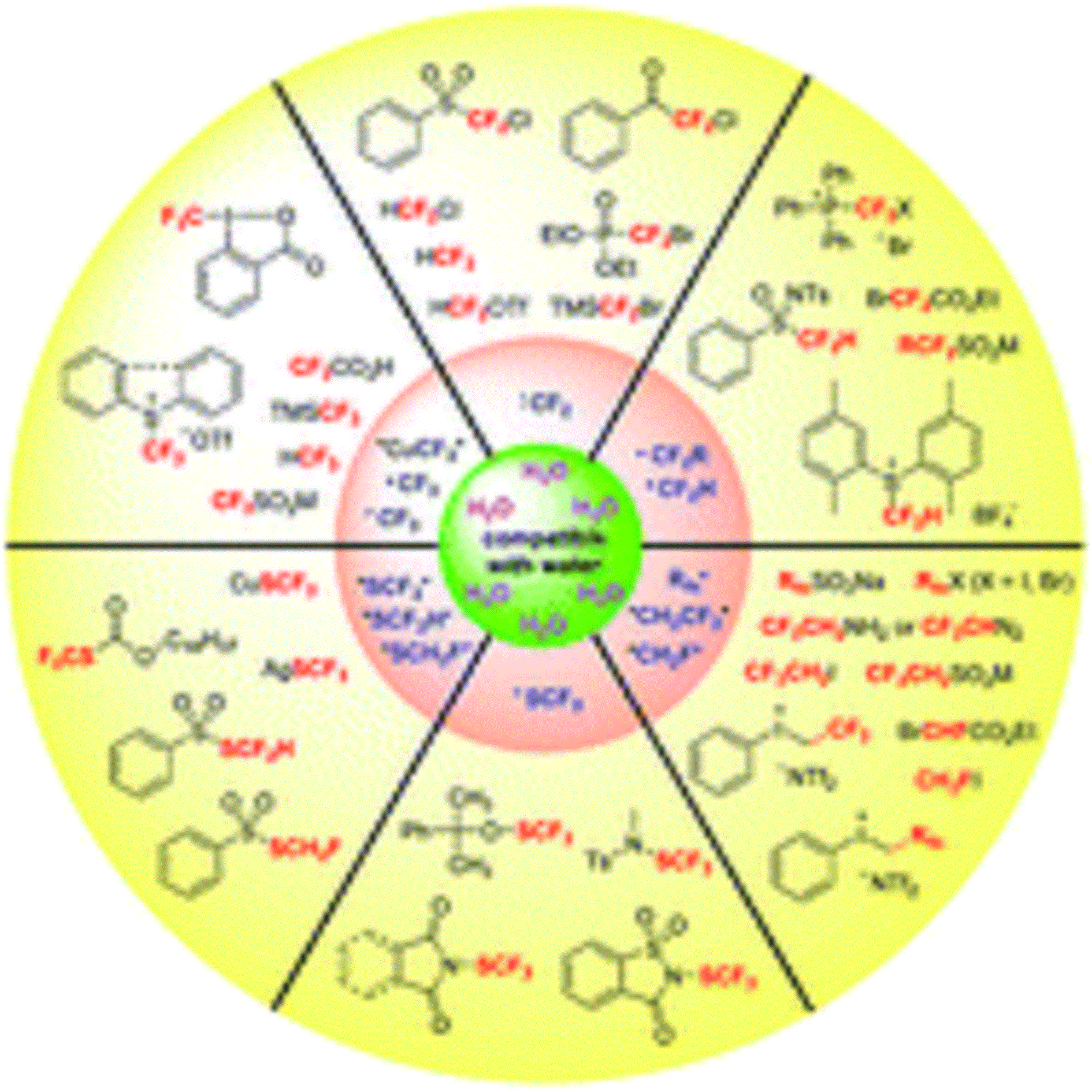
In conclusion, aqueous fluoroalkylation including trifluoromethylation, difluoromethylation, monofluoromethylation, trifluoroethylation, perfluoroalkylation, trifluoromethylthiolation, and difluoromethylthiolation are summarized in this review.
The successful assembly of nucleophilic, radical, and/or electrophilic fluoroalkylation reagents and water in fluoroalkylation reactions opens a new prospect for green chemistry. The valid catalytic systems and the newly developed reagents contribute greatly for the success of the aqueous fluoroalkylation. As a provisional conclusion, the shelf-stable electrophic and radical fluoroalkylation reagents such as “+CF3”, “+CF2H”, “ +CH2CF3”, RfnSO2M (M = Na, 1/2Zn, Cl), RfnX (X = I, Br), and “+ SCF3” reagents are basically compatible with water or aqueous media, which enable a variety of aqueous fluoroalkylation reactions under mild conditions. In the case of nucleophilic fluoroalkylation reagents that are moisture-sensitive (e.g., “−CF3” and “− SCF3” sources), the choice of an appreciate transition-metal partner to stabilize the fluorinated anions is crucial to promote the reaction.
By coupling with the right transition metals, these sensitive fluoroalkylation reagents or intermediates would have sufficient lifetimes to finish the target conversions. Water is abundant and environmentally benign, and it has advantages such as high dielectric constant, large cohesive energy density, and strong hydrogen bonding interaction, which desirably influence the efficiency and selectivity of chemical reactions. In this reviw, water works as a (co)solvent and/or a reactant to facilitate the fluoroalkylation by increasing the dissolving of the reaction participants, providing a proton donor, or behaving as a O-nucleophile.
The fluoroalkylation reactions performed in aqueous media are mild, easily controlled, and environmental friendly, which fit well the principles of green chemistry. Although breakthroughs have been made, siginificant improvement is still neccessary for a wide range of fluoroalkylation reactions. A tough question is whether the direct trifluoromethoxylation can be performed in aqueous conditions, despite the reaction of excess AgOCF3 with α-diazo esters surviving in CH3CN in the presence of residue moisture or a trace amount of D2O (Scheme 120).155 The ionic [Me4N][SCF3] and [Me4N][SeCF3] salts, and their variants containing free − SCF3 or − SeCF3 anions, also encounter similar problems, even through trace of water proved to be essential for the functionalization of α-diazo carbonyls.156,157 The sensitive −XCF3 (X = O, S, Se) anions tend to undergo α-fluorine elimination to generate fluoride ( − F) and carbonic difluoride (CXF2), and the presence of water is generally believed to accelerate this transformation, leading to rapid decomposition of these reagents.
We hope that this review will attract more interests and contributions in the development of aqueous fluoroalkylation with these extraordinary reagents. Aqueous fluoroalkylation methods have changed the way to synthesize fluorinated molecules in terms of the biological and physicochemical properties. Since the aspects of green chemistry have drawn much attention from society, the pursuit of more efficient and milder reaction conditions for greener fluoroalkylation in aqueous media will never be terminated. We hope that this review will serve as a guide to understand and as an appeal to engage in the field of green fluorine chemistry.
To meet the principles of green chemistry, the development of new fluoroalkylation reagents and efficient catalytic systems will be continuously vital for the mild and environment-benign fluoroalkylation. It is anticipated that a growing number of green fluoroalkylation methodologies in aqueous media will arise in the near future.













Sorry, the comment form is closed at this time.