Feb 232018
(Z and E)-4-(Methylamino)-3-(4-nitrobenzoyl)-2-oxobut-3-enoic Acid Ethyl Ester (2a)
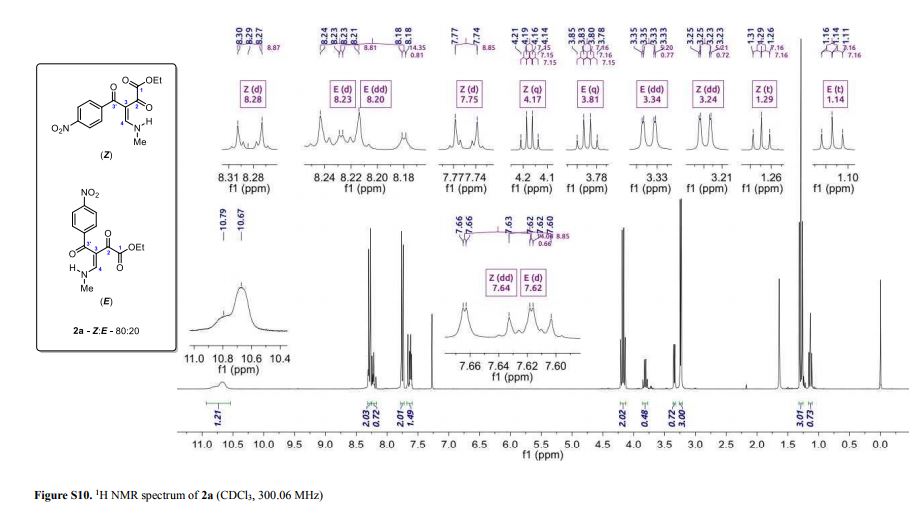
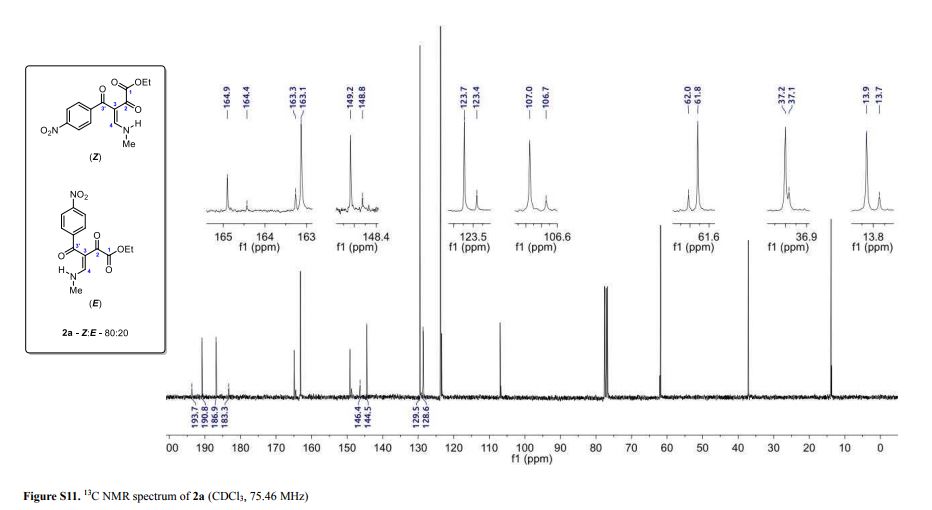
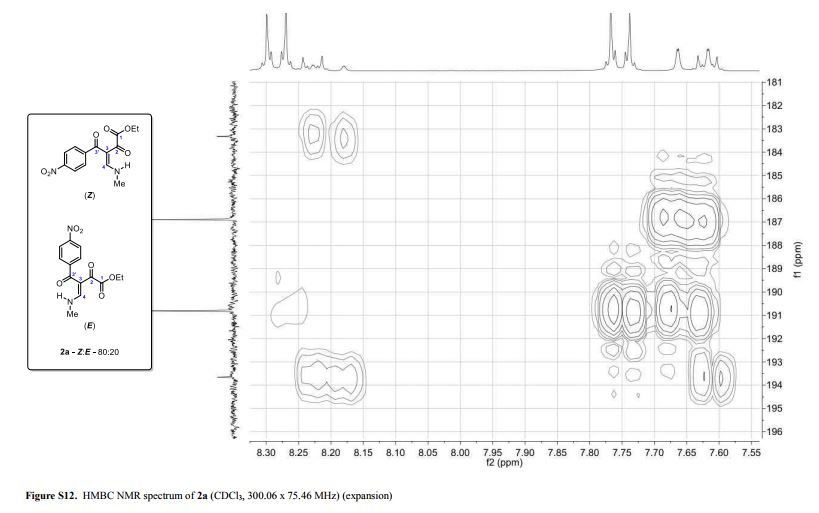
Light yellow solid; yield: 0.276 g (90%); Z/E ratio in CDCl3: 80/20; mp 143.8–145.3 °C;
1H NMR (300.06 MHz, CDCl3) δ (ppm) (Z) 1.29 (t, 3H, J = 7.2 Hz, O–CH2–CH3), 3.24 (dd, 3H, J = 5.2, 0.7 Hz, NH-CH3), 4.17 (q, 2H, J = 7.2 Hz, O–CH2-CH3), 7.64 (dd, 1H, J = 14.1, 0.7 Hz, H4), 7.75 (d, 2H, J = 8.8 Hz, 4-NO2C6H4), 8.28 (d, 2H, J = 8.9 Hz, 4-NO2C6H4), 10.67 (bs, 1H, NH); (E) 1.14 (t, 3H, J = 7.2 Hz, O–CH2–CH3), 3.34 (dd, 3H, J = 5.2, 0.8 Hz, NH-CH3), 3.81 (q, 2H, J = 7.2 Hz, O–CH2-CH3), 7.62 (d, 2H, J = 8.8 Hz, 4-NO2C6H4), 8.20 (dd, 1H, J = 14.3, 0.8 Hz, H4), 8.23 (d, 2H, J= 8.8 Hz, 4-NO2C6H4), 10.79 (bs, 1H, NH);
13C NMR (75.46 MHz, CDCl3) δ (ppm) (Z) 13.9 (O–CH2–CH3), 37.2 (NH-CH3), 61.8 (O-CH2–CH3), 107.0 (C3), 123.7, 129.5, 144.5, 149.2 (4-NO2C6H4), 163.1 (C4), 164.9 (COOEt), 186.9 (C2), 190.8 (C3′); (E) 13.7 (O–CH2–CH3), 37.1 (NH-CH3), 62.0 (O-CH2–CH3), 106.7 (C3), 123.4, 128.6, 146.4, 148.8 (4-NO2C6H4), 163.3 (C4), 164.4 (COOEt), 183.3 (C2), 193.7 (C3′);
HRMS (ESI+): calcd for C14H15N2O6+, [M+H]+: 307.0925, found 307.0938.
J. Org. Chem., 2017, 82 (23), pp 12590–12602
DOI: 10.1021/acs.joc.7b02361
//////////////https://pubs.acs.org/doi/abs/10.1021/acs.joc.7b02361













Sorry, the comment form is closed at this time.