DOI: 10.1039/C7GC03437G, Communication
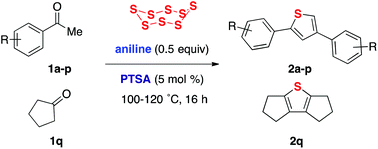
An aniline/acid-catalyzed method for constructing thiophenes 2 from inexpensive ketones 1 and elemental sulfur is reported.
Sulfurative self-condensation of ketones and elemental sulfur: a three-component access to thiophenes catalyzed by aniline acid–base conjugate pairs
Abstract
A sulfurative self-condensation method for constructing thiophenes 2 by a reaction between ketones 1 and elemental sulfur is reported. This reaction, which is catalyzed by anilines and their salts with strong acids, starts from readily available and inexpensive materials, and releases only water as a by-product.
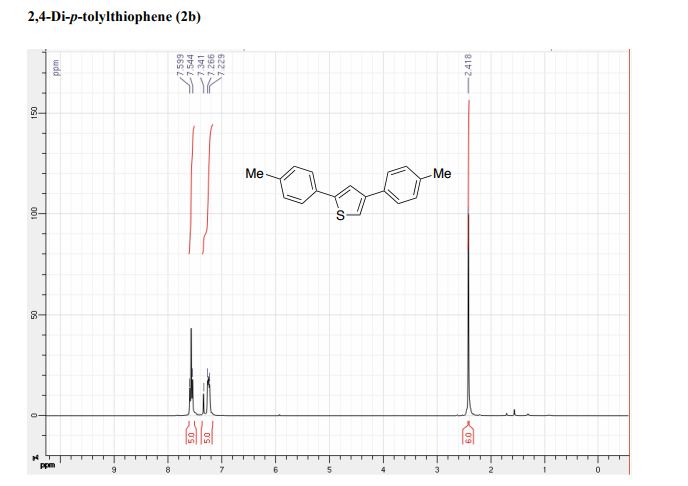
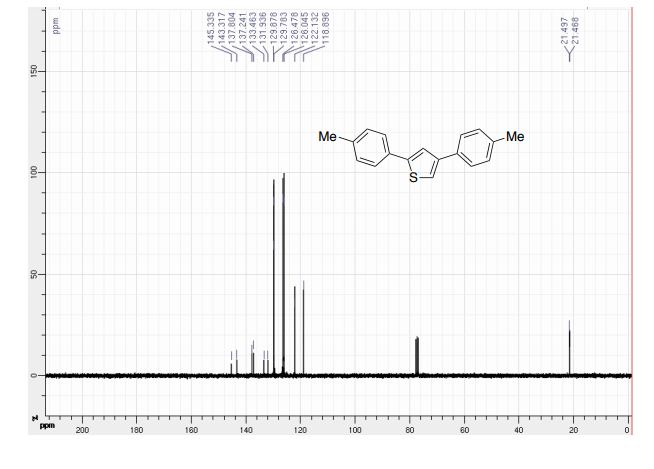
2,4-Di-p-tolylthiophene (2b)2
2 M. Arisawa, T. Ichikawa, and M. Yamaguchi, Chem. Commun. 2015, 51, 8821
Eluent heptane:toluene 9:1. 190 mg, 72%.
1 H NMR (300 MHz, CDCl3) δ 7.60-7.54 (m, 5H), 7.34 (s, 1H), 7.27-7.23 (m, 4H), 2.42 (s, 6H).
13C NMR (75 MHz, CDCl3) δ 145.3, 143.3, 137.8, 137.2, 133.5, 131.9, 129.9, 129.8, 126.5, 126.0, 122.1, 118.9, 21.5, 21.5.


Binh Thanh Nguyen
CV Binh Nguyen
CNRS Research Associate CR1 ( ORCID , ResearchGate )
ICSN-CNRS Bât. 27
1, avenue de la Terrasse
91190 Gif-sur-Yvette France
thanh-binh.nguyen_at_cnrs.fr
+33 1 69 82 45 49
![]() Education and work experience2015: Habilitation to Direct Research (HDR)
Education and work experience2015: Habilitation to Direct Research (HDR)
2011 – present: CNRS research associate at ICSN – Paris-Saclay University
2009 – 2011: Post-doctoral Fellow at ICSN (Dr. Françoise Guéritte and Dr. Qian Wang)
2003 – 2006: Ph.D. student at the UCO2M Organic Synthesis Laboratory (University of Maine, Le Mans, France, Dr. Gilles Dujardin, Dr. Arnaud Martel, Professor Robert Dhal)
![]() Research Interests
Research Interests
Green chemistry (Atom, step and redox economic transformation), green synthetic tools: O2, S8, photochemistry, iron catalyst
Elemental sulfur as a synthetic tool (building block, oxidant, reductant, catalyst)
Iron-sulfur catalysts
Heterocycle synthesis
![]() Scientific Communications
Scientific Communications
47 publications
![]() Selected recent publications ( complete list )
Selected recent publications ( complete list )
[1] Adv. Synth. Catal. 2017 , 359 , 1106.
[2] Asian J. Org. Chem. 2017 , 6 , 477.
[3] Org. Lett. 2016 , 18 , 2177.
[4] Org. Process Res. Dev. 2016 , 20 , 319.
[5] Angew. Chem. Int. Ed. 2014 , 53 , 13808.
[6] J. Am. Chem. Soc. 2013 , 135 , 118.

///////////














Sorry, the comment form is closed at this time.