DOI: 10.1039/C7GC01874F, Communication
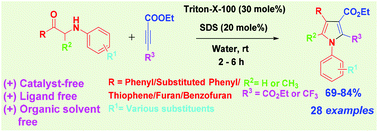
An efficient and metal-free method has been developed for the synthesis of polysubstituted pyrrole derivatives with combination of sodium dodecyl sulphate (SDS) and Triton X-100 surfactants using water as a solvent at room temperature in 2-6 h and under microwave conditions (10 min) with good to excellent yields.
Metal-free synthesis of polysubstituted pyrroles using surfactants in aqueous medium
Dr. Narender Tadigoppula
Principal Scientist
Medicinal & Process Chemistry
Central Drug Research Institute
India
Dr. Narender Tadigoppula is currently principal scientist in the department of medicine chemistry central drug research institute. He published more than 30 research articles. His major major research activities are identification of biologically active lead molecules through activity guided fraction and isolation work on the medicinal plants, marine organisms and microorganisms for metabolic diseases (hyperglycemia, dyslipidemia), parasitic diseases (leishmania and malaria), cancer etc., and chemical transformation of natural products of biological importance to improve their potency. We synthesize these biologically active lead molecules and their analogues in our laboratory. We have identified several lead molecules from the Indian medicinal plants for various disease areas as described below and further work is in progress to develop natural products based drugs.
Abstract
An efficient and metal-free method has been developed for the synthesis of polysubstituted pyrrole derivatives via intermolecular cycloaddition of substituted 1-phenyl-2-(phenylamino)-ethan-1-one/1-phenyl-2-(phenylamino)-propan-1-ones/2-((4-methoxyphenyl)amino)-1-(thiophen-2-yl)ethan-1-one/1-(furan-2-yl)-2-((4-methoxyphenyl)amino)ethan-1-one/1-(benzofuran-3-yl)-2-((4-methoxyphenyl)amino)ethan-1-one and dialkyl acetylene dicarboxylate/ethylbutynoate in the presence of a combination of sodium dodecyl sulphate (SDS) and Triton X-100 surfactants using water as a solvent at room temperature in 2–6 h under microwave conditions (10 min) with good to excellent yields.
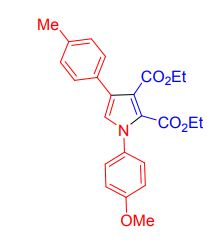
Diethyl-1-(4-methoxyphenyl)-4-(p-tolyl)-1H-pyrrole 2,3dicarboxylate
white solid, yield 77%, mp 128-130 ;
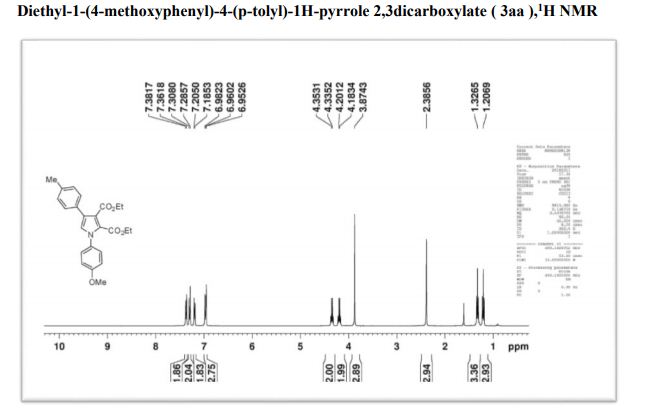
1H NMR (400 MHz, CDCl3) δ 7.38(d, J = 8.2,2H), 7.31 (d, J = 7.9, 2H), 7.21 (d, J = 7.12, 2H), 6.99-6.96 (m, 3H), 4.31 (q, J = 7.2 Hz, 2H), 4.12 (q, J = 7.6Hz, 2H), 3.88 (s, 3H), 2.38 (s, 3H), 1.31 (t, J = 7.9Hz, 3H), 1.19 (t, J = 7.5Hz, 3H) ;
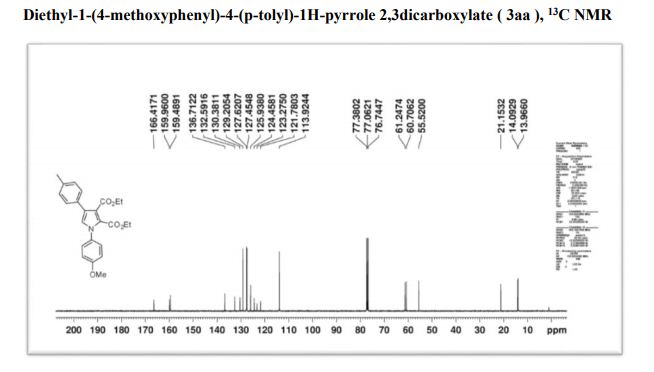
13C NMR (100 MHz, CDCl3) δ 166.3, 159.9, 149.0, 148.8, 136.7, 132.6, 130.3, 129.2, 127.6, 125.8, 124.5, 123.4, 121.5, 118.3, 110.5, 110.2, 61.2, 60.7, 56.0, 21.1, 14.0, 13.9.
IR (KBr) ṽ (cm-1): 2981.9, 1717.9, 1514.1, 1419.2, 1381.3, 1245.0, 1175.9, 1226.7, 1043.6, 835.7, 755.3, 663.
HRESIMS: m/zcalcd for [M+H]+ C24H26NO5 408.1805 found 408.1845.
/////////////
O=C(OCC)c2c(c(cn2c1ccc(OC)cc1)c3ccc(C)cc3)C(=O)OCC















Sorry, the comment form is closed at this time.