DOI: 10.1039/C7GC02190A, Paper
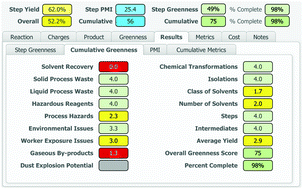
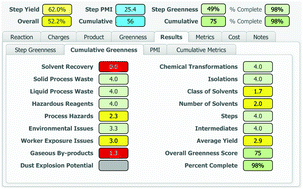
A process greenness scorecard has been developed that provides a comprehensive assessment of greenness aspects not encompassed by mass-based metrics, including environmental, health and safety impacts, in order to facilitate the design of greener, more benign and inherently safer processes.
The content of this RSS Feed (c) The Royal Society of Chemistry

Melanie Miller

Head of API Operations, Pharmaceutical Development
Bristol Myers Squibb
New Brunswick, New Jersey
Leads manufacturing operations to deliver small molecule active pharmaceutical ingredients for investigational medicines. Scope includes all R&D API manufacturing operations within a global external and internal manufacturing network supporting delivery of small molecules, antibody-drug conjugates, peptides and oligonucleotides.
Design and evolution of the BMS process greenness scorecard
Jason Sweeney
Associate Director at Bristol-Myers Squibb
Abstract
An accurate and comprehensive assessment of the environmental, health and safety impacts of a chemical process is critical to the design and implementation of greener, more benign and inherently safer processes. Over the past 15 years at BMS, we have developed a Process Greenness Scorecard to capture and analyse a number of metrics and attributes for each step in the synthetic sequence used to produce an API. This manuscript describes the design and evolution of the scoring methodology and implementation of the resulting scorecard, from an initial Excel-based tool to the current web-based format.

-
 Associate Director, Process Chemistry
Associate Director, Process ChemistryCompany NameTakeda
Dates EmployedJul 2017 – Present
Employment Duration4 mos
LocationCambridge, MA
-

Principal Scientist
Company NameBristol-Myers Squibb
Dates Employed2013 – 2017
Employment Duration4 yrs
LocationGreater New York City Area
• Project leader for two high priority small molecule oncology programs, delivering preclinical supplies for five separate analogues (three separate chemotypes) at an external CMO at least one month prior to candidate nomination. Developed a new chemical route for the most complex analogue to overcome poor efficiency (key fragment improved from 1.4% yield in 8 steps to 33% yield in 5 steps).
• Chemistry lead for a highly accelerated oncology program, responsible for the late-phase development and external execution of three complex regulatory starting materials. Invented a new synthetic route to circumvent a key scale-up liability, while removing numerous bottlenecks.
• Project leader for a biologics/small molecule conjugate PET tracer. Developed bio-orthogonal chemistry to prepare and purify the cold PET tracer analogue needed for IND toxicology studies. Key thought leader in developing new CMC strategies utilized by subsequent PET tracer teams.
• CMC leader for diabetes and fibrosis assets. Led cross-functional CMC team and Exploratory Development team through a very complex tautomer challenge. Strategically laid out and executed plans demonstrating that the tautomers posed no risk to patient safety and effectively communicated this to the FDA. Guided team to achieve key program milestone of First in Human.
• Co-invented a novel, non-chromatographic approach to purification of macro-cyclic peptides.
• Co-chair of the ACS Green Chemistry Institute Pharmaceutical Roundtable, responsible for implementation of strategic priorities and management of a $375k budget.
• Team lead for BMS corporate sustainability 2015 and 2020 goals to integrate sustainability throughout development and commercialization.
• Supervision of Ph.D. level direct reports. Committed to the professional development of project teams and direct reports, helping refine their skills, obtain promotions, and identify new roles that meet their career aspirations and passion.

Eric Simmons

William Fleming
Bristol-Myers Squibb

////////////////Bristol-Myers Squibb, bms, green
more…………..
A data-driven strategy for predicting greenness scores, rationally comparing synthetic routes and benchmarking PMI outcomes for the synthesis of molecules in the pharmaceutical industry†
Jun Li , Eric M. Simmons and Martin D. Eastgate *
Chemical and Synthetic Development, Bristol-Myers Squibb, 1 Squibb Drive, New Brunswick, NJ 08903, USA. E-mail: martin.eastgate@bms.com
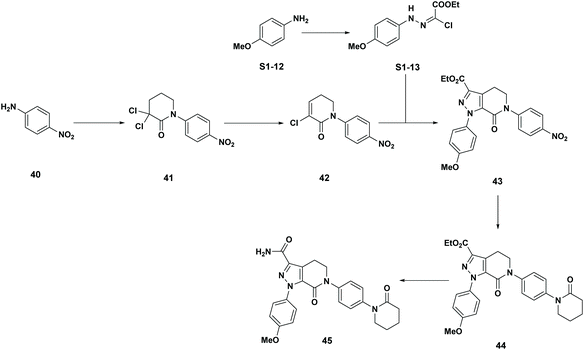
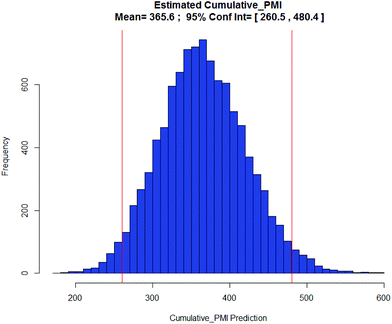
Apixaban: Our final case study is apixaban (45), an orally bioavailable inhibitor of blood coagulation factor Xa, developed for thrombotic diseases and commercialized as Eliquis (Scheme 6).17 This highly optimized process evolved through multiple rounds of development and the data reported is taken from the validation campaign, thus ready for product launch. The actual cumulative PMI for the overall process was 197, which is significantly below the lower end of the 95% confidence interval for the predicted cumulative PMI (Fig. 14). In essence, it is lower than 99.9% of the similar chemistries executed on scale at different development stages. This is the one of a few commercial assets in our current database, and while obviously efficient, this score should be viewed with the perspective that most of the data available to us in this proof of concept study is in the development phase, and thus encompasses a wide range of optimization levels. However, in order to compare more globally, more data, from more companies, and across all phases of development is needed.
 |
||
| Scheme 6 Apixaban synthetic route in validation campaign. | ||
| Fig. 14 Predicted apixaban cumulative PMI with mean 366 and 95% CI between 261 and 480. |
“ALL FOR DRUGS” CATERS TO EDUCATION GLOBALLY, No commercial exploits are done or advertisements added by me. This is a compilation for educational purposes only. P.S. : The views expressed are my personal and in no-way suggest the views of the professional body or the company that I represent














Sorry, the comment form is closed at this time.