
A fully continuous-flow diazotization–hydrolysis protocol has been developed for the preparation of p-cresol. This process started from the diazotization of p-toluidine to form diazonium intermediate. The reaction was then quenched by urea and subsequently followed by a hydrolysis to give the final product p-cresol. Three types of byproducts were initially found in this reaction sequence. After an optimization of reaction conditions (based on impurity analysis), side reactions were eminently inhibited, and a total yield up to 91% were ultimately obtained with a productivity of 388 g/h. The continuous-flow methodology was used to avoid accumulation of the highly energetic and potentially explosive diazonium salt to realize the safe preparation for p-cresol.
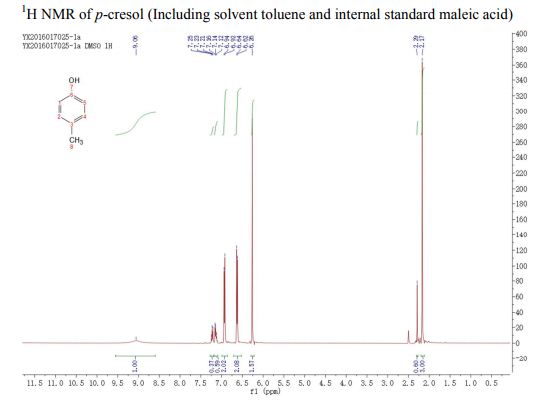
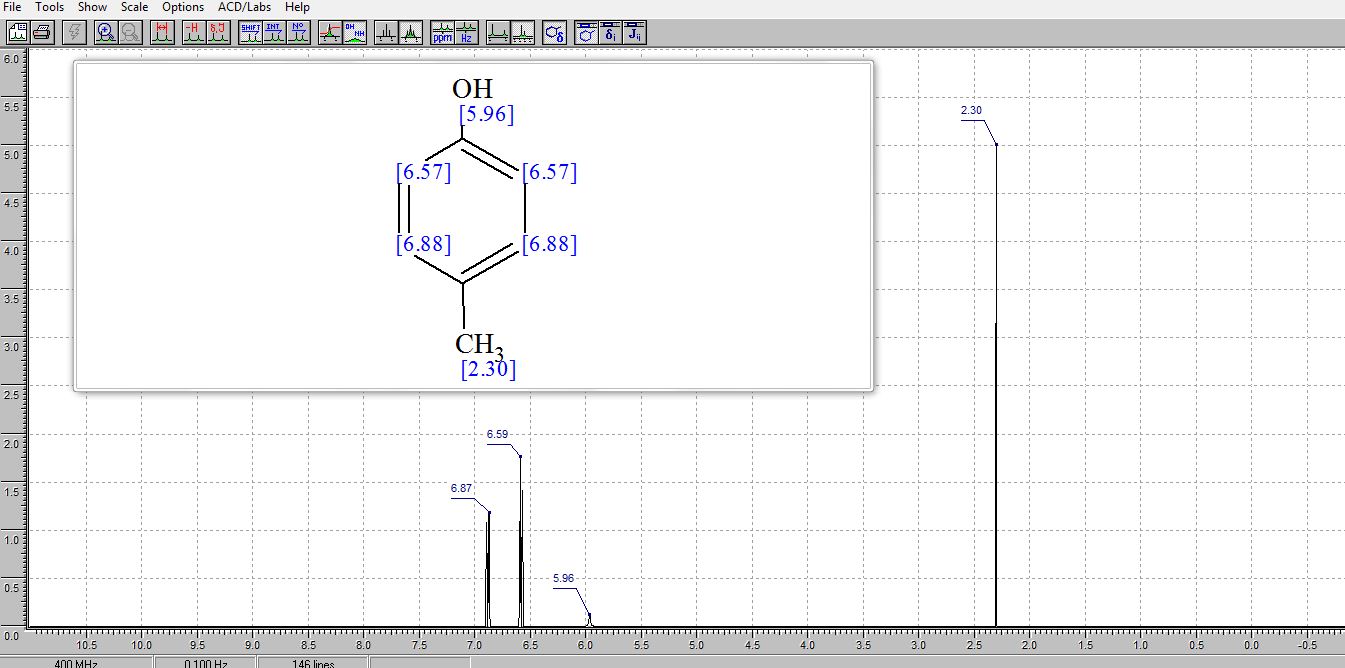
. 1H NMR (400 MHz, (CD3)2SO) δ/ppm: 9.06 (br s, 1H, −OH), 6.94 (d, J = 8.0 Hz, 2H, Ar–H), 6.62 (d, J = 8.0 Hz, 2H, Ar–H), 2.17 (s, 3H, −CH3).
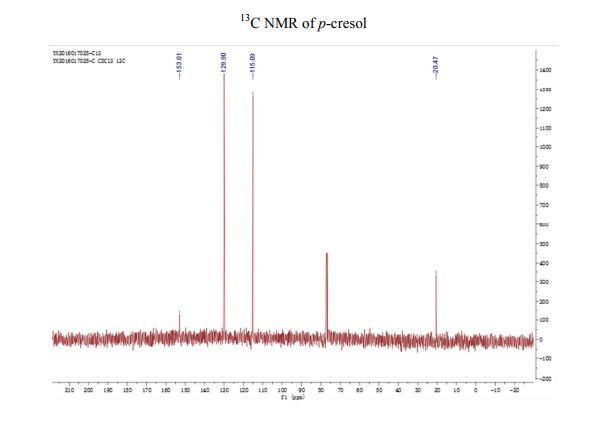
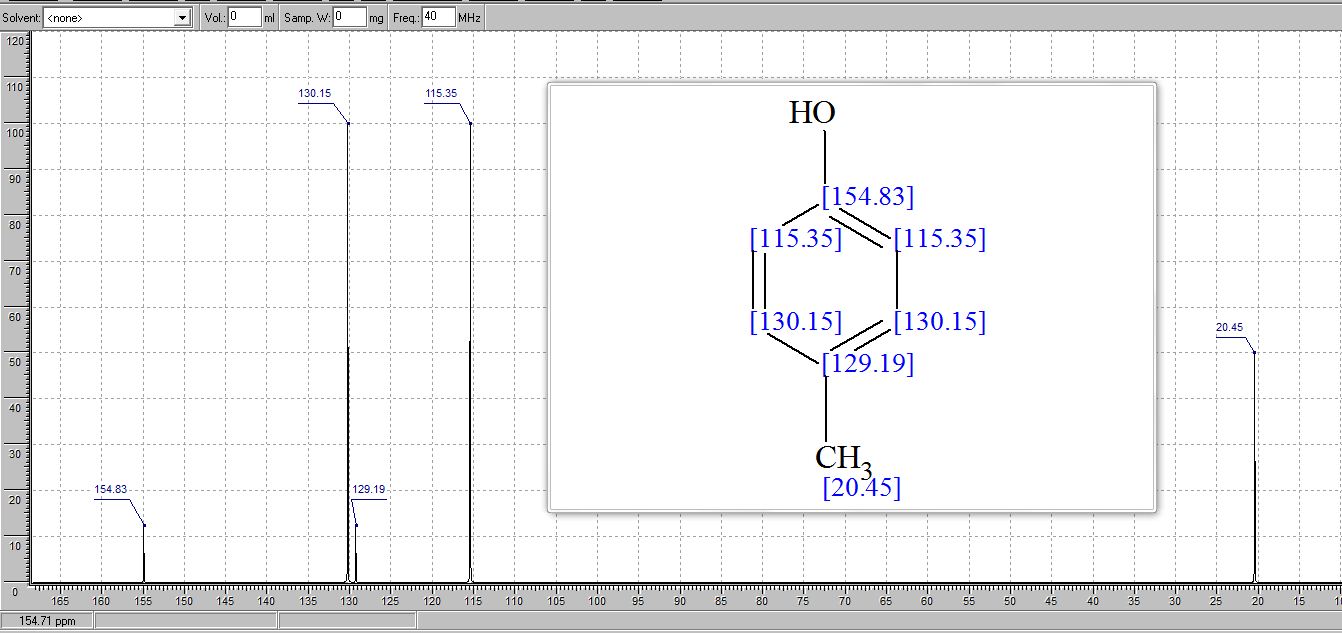
13C NMR (CDCl3) δ/ppm: 153.0, 129.9, 115.1, 20.5.
Literature data:(3b) 1H NMR (300 MHz, CDCl3) δ/ppm: 7.03 (d, J = 8.2 Hz, 2H), 6.73 (dd, J = 8.2, 2.0 Hz, 2H), 4.75 (s, 1H, OH), 2.27 (s, 3H, CH3).
13C NMR (CDCl3) δ/ppm: 153.2, 130.2, 115.2, 20.6.
3(b) Taniguchi, T.; Imoto, M.; Takeda, M.; Nakai, T.; Mihara, M.; Iwai, T.; Ito, T.; Mizuno, T.; Nomoto, A.; Ogawa, A. Heteroat. Chem. 2015, 26, 411– 416 DOI: 10.1002/hc.21275
A Fully Continuous-Flow Process for the Synthesis of p-Cresol: Impurity Analysis and Process Optimization
http://pubs.acs.org/doi/full/10.1021/acs.oprd.7b00250
NMR PREDICT














Sorry, the comment form is closed at this time.