Advances in indoleamine 2,3-dioxygenase 1 medicinal chemistry
DOI: 10.1039/C7MD00109F, Review Article
 Open Access
Open Access This article is licensed under a Creative Commons Attribution 3.0 Unported Licence.
This article is licensed under a Creative Commons Attribution 3.0 Unported Licence.Structure-function relationships of IDO1 and structure-activity relationships of inhibitors are discussed with an outlook on next generation IDO1 ligand.
MedChemComm
Advances in indoleamine 2,3-dioxygenase 1 medicinal chemist
Abstract
Indoleamine 2,3-dioxygenase 1 (IDO1) mediates multiple immunoregulatory processes including the induction of regulatory T cell differentiation and activation, suppression of T cell immune responses and inhibition of dendritic cell function, which impair immune recognition of cancer cells and promote tumor growth. On this basis, this enzyme is widely recognized as a valuable drug target for the development of immunotherapeutic small molecules in oncology. Although medicinal chemistry has made a substantial contribution to the discovery of numerous chemical classes of potent IDO1 inhibitors in the past 20 years, only very few compounds have progressed in clinical trials. In this review, we provide an overview of the current understanding of structure–function relationships of the enzyme, and discuss structure–activity relationships of selected classes of inhibitors that have shaped the hitherto few successes of IDO1 medicinal chemistry. An outlook opinion is also given on trends in the design of next generation inhibitors of the enzyme.
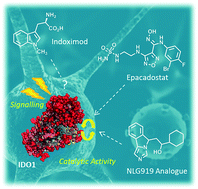
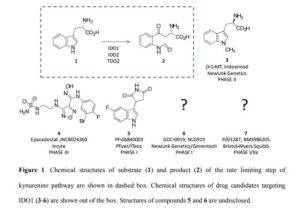
Introduction Indoleamine 2,3-dioxygenases (IDOs) are heme-containing proteins that catalyze the oxidative cleavage of the indole ring of tryptophan (L-Trp, 1) to produce N-formyl kynurenine (2) in the first rate limiting step of the kynurenine pathway (Figure 1).1,2 The family includes two related enzymatic isoforms, namely IDO1 and IDO2, sharing ∼60% of sequence similarity and featuring distinct biochemical features.3,4 A third enzyme of the family is the tryptophan-2,3-dioxygenase (TDO2) which is structurally unrelated to IDO1 and IDO2 and is endowed with a more stringent substrate specificity for L-Trp.5 Although TDO2 is expressed almost exclusively in hepatocytes where it regulates L-Trp catabolism in response to the diet, IDO1 and IDO2 are widely expressed in macrophages and dendritic cells exerting immunoregulatory functions.6 These are accomplished through two major mechanisms including depletion of tryptophan and production of bioactive metabolites along the kynurenine pathway. Specifically, the first mechanism postulates that raising levels of Interferon-γ (IFN-γ) induce IDO1 expression in macrophages and dendritic cells during pathogen infection, leading to consumption of L-Trp and growth arrest of pathogens, whose diet is sensitive to this essential nutrient.7 The second mechanism grounds on production of kynurenine metabolites that bind to the aryl hydrocarbon receptor (AhR), activating signaling pathways that enhance immune tolerance.8-10 Among the three proteins, IDO1 is the most characterized enzyme and in recent years a second signal-transducing function was revealed for this protein.11,12 In particular, this signalling function relies on the presence of two immunoreceptor tyrosine-based inhibitory motifs (ITIMs) in the non-catalytic domain of IDO1.13 The immunosuppressive cytokine transforming growth factor-β (TGF-β) stimulates phosphorylation of ITIMs by Sarcoma-family (Src-family) kinases and consequent interaction of the phosphorylated enzyme with Src Homology 2 domain Phosphatase-1 (SHP-1) and Src Homology 2 domain Phosphatase-2 (SHP-2), eventually leading to long-term expression of IDO1 and immune tolerance. Conversely, in pro-inflammatory environmental conditions, increasing levels of interleukin-6 (IL-6) trigger the interaction of
phosphorylated IDO1 with suppressor of cytokine signalling 3 (SOCS3) that tags the enzyme for proteasome degradation, shortening IDO1’s half-life and promoting inflammatory response.14 The breakthrough discovery that IDO1 plays a crucial role in the maintenance of maternal immune tolerance ushered in a great deal of interest on the enzyme, by then considered a master regulatory hub of immunosuppressive pathways in pregnancy, autoimmune diseases, chronic inflammation, and cancer.15 In this framework, elevated levels of IDO1 expression found in several tumour cells were associated to the participation of the enzyme in the tumor immuno-editing process which sets up immune tolerance to tumor antigens.16,17 On this basis, academic groups and pharmaceutical companies have been engaged in the development of IDO1 inhibitors.18 Although part of these efforts has proved successful, with a large array of potent and selective inhibitors being disclosed in literature and patent applications, only few compounds have hitherto entered clinical trials (3-7, Figure 1).2,19-22 At this regard, some studies have highlighted challenges in the development of enzyme inhibitors mostly due to redox properties of the enzyme that may account for unspecific mechanism of inhibition of many compounds discovered in preclinical studies.23,24 Starting with an overview on the architecture of IDO1 and its structure-function relationships, in this article we discuss selected classes of inhibitors that have shaped advances in the medicinal chemistry of IDO1, providing outlooks on future trends in the design of next generation compounds.


//////////

















Sorry, the comment form is closed at this time.