Jul 142017
Catalytic carbonyl hydrosilylations via a titanocene borohydride-PMHS reagent system
Catal. Sci. Technol., 2017, Advance Article
DOI: 10.1039/C7CY01088E, Paper
Godfred D. Fianu, Kyle C. Schipper, Robert A. Flowers II
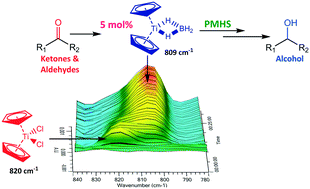
Catalytic amounts of titanocene(III) borohydride, generated under mild conditions from commercially available titanocene dichloride, in concert with a stoichiometric hydride source is shown to effectively reduce aldehydes and ketones to their respective alcohols in aprotic media.
Catalytic amounts of titanocene(III) borohydride, generated under mild conditions from commercially available titanocene dichloride, in concert with a stoichiometric hydride source is shown to effectively reduce aldehydes and ketones to their respective alcohols in aprotic media.
-
Catalysis Science & Technology
Catalytic carbonyl hydrosilylations viaa titanocene borohydride–PMHS reagent system
Abstract
Reduction of a wide range of aldehydes and ketones with catalytic amounts of titanocene borohydride in concert with a stoichiometric poly(methylhydrosiloxane) (PMHS) reductant is reported. Preliminary mechanistic studies demonstrate that the reaction is mediated by a reactive titanocene(III) complex, whose oxidation state remains constant throughout the reaction.

Godfred Fianu
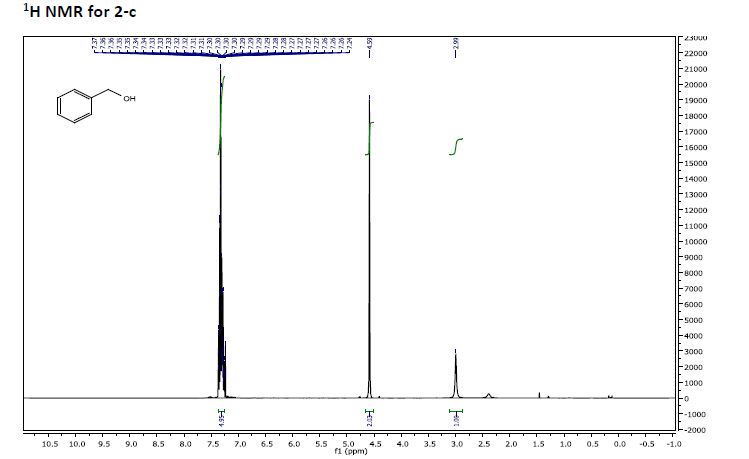
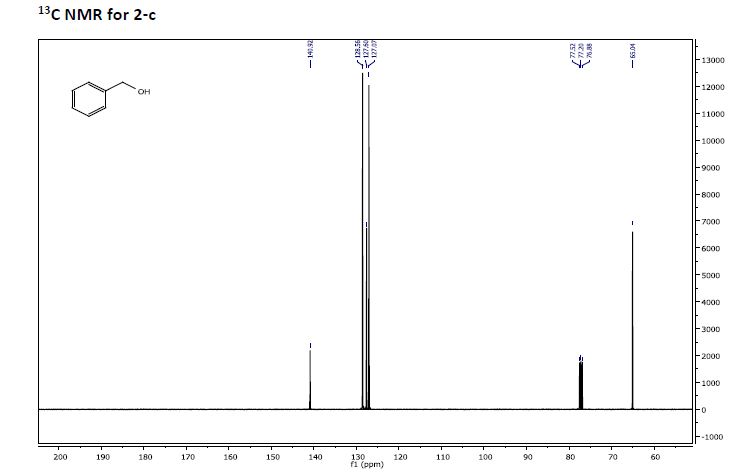
Phenyl methanol (2-c)
Phenyl methanol (2-c) was prepared from benzaldehyde (1-c) by the procedure outlined
in GP1. NMR analysis showed 100% conversion in 1 hour. 86% isolated yield of alcohol
product was obtained after complete workup.
Phenyl methanol (2-c) was prepared from benzaldehyde (1-c) by the procedure outlined
in GP1. NMR analysis showed 100% conversion in 1 hour. 86% isolated yield of alcohol
product was obtained after complete workup.
1H NMR (400 MHz, CDCl3) δ 7.37 – 7.26 (m,5H), 4.59 (s, 2H), 2.99 (s, 1H).
13C NMR (101 MHz, CDCl3) δ 140.92, 128.56, 127.60, 127.07,77.52, 77.20, 76.88, 65.04.
//////////////















Sorry, the comment form is closed at this time.