Jun 052017

The first continuous flow synthesis of C8–C16 alkane fuel precursors from biobased platform molecules is reported. TBD (1,5,7-triazabicyclo[4.4.0]dec-5-ene) was found to be a recyclable and highly efficient organic base catalyst for the aldol condensation of furfural with carbonyl compounds, and the selectivity of mono- or difuryl product can be easily regulated by adjusting the molar ratio of substrates. By means of flow technique, a shorter reaction time, satisfactory output, and continuous preparation are achieved under the present procedure, representing a significant advance over the corresponding batch reaction conditions.
Continuous Microflow Synthesis of Fuel Precursors from Platform Molecules Catalyzed by 1,5,7-Triazabicyclo[4.4.0]dec-5-ene
† College of Biotechnology and Pharmaceutical Engineering, Nanjing Tech University, Nanjing 211816, China
‡National Engineering Technique Research Center for Biotechnology, Nanjing 211816, China
§Jiangsu National Synergetic Innovation Center for Advanced Materials (SICAM), Nanjing 211816, China
∥State Key Laboratory of Motor Vehicle Biofuel Technology, Nanyang 473000, China
Org. Process Res. Dev., Article ASAP
DOI: 10.1021/acs.oprd.7b00141
*E-mail: zhucj@njtech.edu.cn. Phone/Fax: +86 25 58139389.
1-(furan-2-yl)-2-methylpent-1-en-3-one
1a
3-pentanone (100 mmol, 8.6 g) and furfural (100 mmol, 9.6 g) were diluted with MeOH-H2O to 40 mL in stream 1, catalyst TBD (10 mmol, 1.39 g) were diluted with MeOH-H2O (v/v = 1/1) to 40 mL in stream 2, the two streams was purged in a 0.2 mL/min speed into slit plate mixer and at the 353 K passed tubing reactor. Finally, the product was extracted with EtOAc and water, the obtained organic layer was evaporated and purified by silica gel flash chromatography (25:1 hexane-EtOAc) to provide the analytically pure product for further characterization, the aqueous phase was collected and reused.According to the general procedure afforded 14.92 g (91%) of product 1a, isolated as pale yellow oil;
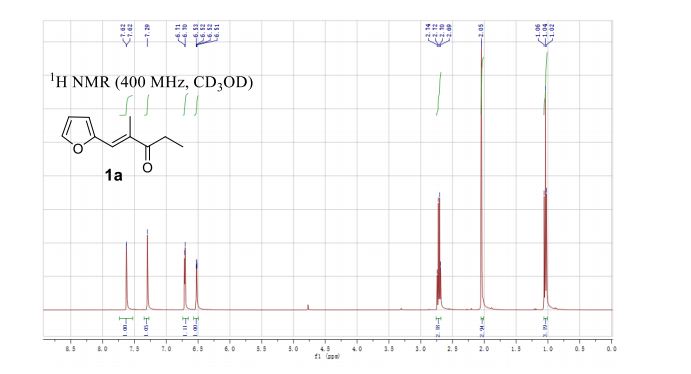
1H NMR (400 MHz, CD3OD) δ 7.62 (d, J = 1.4 Hz, 1H), 7.29 (s, 1H), 6.71 (d, J = 3.5 Hz, 1H), 6.52 (dd, J = 3.4, 1.8 Hz, 1H), 2.71 (q, J = 7.3 Hz, 2H), 2.05 (s, 3H), 1.04 (t, J = 7.3 Hz, 3H).
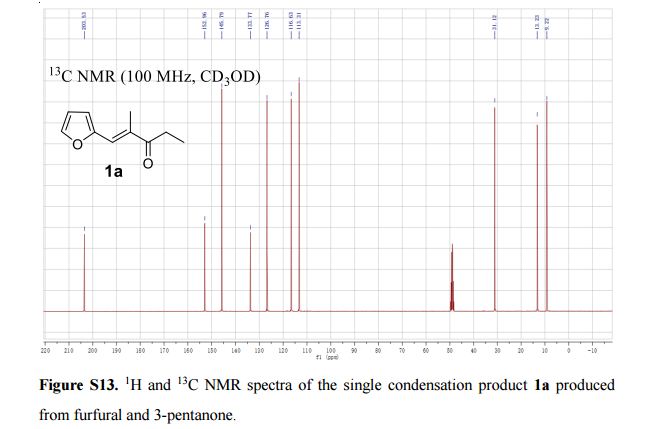
13C NMR (100 MHz, CD3OD) δ 203.5, 153.0, 145.8, 133.8, 126.8, 116.6, 113.3, 31.1, 13.2, 9.2.
////////













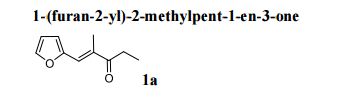




 LinkedIn
LinkedIn Facebook
Facebook Twitter
Twitter GooglePlus
GooglePlus
Sorry, the comment form is closed at this time.