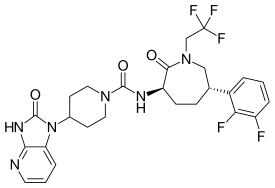
Telcagepant, MK-0974
- Molecular FormulaC26H27F5N6O3
- Average mass566.523 Da
- OriginatorMerck & Co
- ClassAntimigraines; Piperidines
- Mechanism of ActionCalcitonin gene-related peptide receptor antagonists
Migraine is a neurovascular disorder characterized by severe, debilitating, and throbbing unilateral headache. Though a leading cause of disability, it is a highly prevalent disease with a clear unmet medical need. With the significant progress achieved in the field of pathophysiology in the past decades, to date, it is well recognized that the neuropeptide calcitonin gene-related peptide (CGRP), which is expressed mainly in the central and peripheral nervous system, plays a crucial role in migraine. Antagonism of CGRP receptors, as a potential new therapy for the treatment of migraine, could offer the advantage of avoiding the cardiovascular liabilities associated with other existing antimigraine therapies.
Telcagepant (INN) (code name MK-0974) is a calcitonin gene-related peptide receptor antagonist which was an investigational drug for the acute treatment and prevention of migraine, developed by Merck & Co. In the acute treatment of migraine, it was found to have equal potency to rizatriptan[1] and zolmitriptan[2] in two Phase III clinical trials. The company has now terminated development of the drug.
Mechanism of action
The calcitonin gene-related peptide (CGRP) is a strong vasodilator primarily found in nervous tissue. Since vasodilation in the brain is thought to be involved in the development of migraine and CGRP levels are increased during migraine attacks, this peptide may be an important target for potential new antimigraine drugs.
Telcagepant acts as a calcitonin gene-related peptide receptor (CRLR) antagonist and blocks this peptide. It is believed to constrict dilated blood vessels within the brain.[3]
Termination of a clinical trial
A Phase IIa clinical trial studying telcagepant for the prophylaxis of episodic migraine was stopped on March 26, 2009 after the “identification of two patients with significant elevations in serum transaminases”.[4] A memo to study locations stated that telcagepant had preliminarily been reported to increase the hepatic liver enzyme alanine transaminase (ALT) levels in “11 out of 660 randomized (double-blinded) study participants.” All study participants were told to stop taking the medication.[5]
On July 29, 2011, it was reported that Merck & Co. were discontinuing the clinical development program for telcagepant. According to Merck, “[t]he decision is based on an assessment of data across the clinical program, including findings from a recently completed six-month Phase III study”.[6]
CLIP



CLIP
CLIP
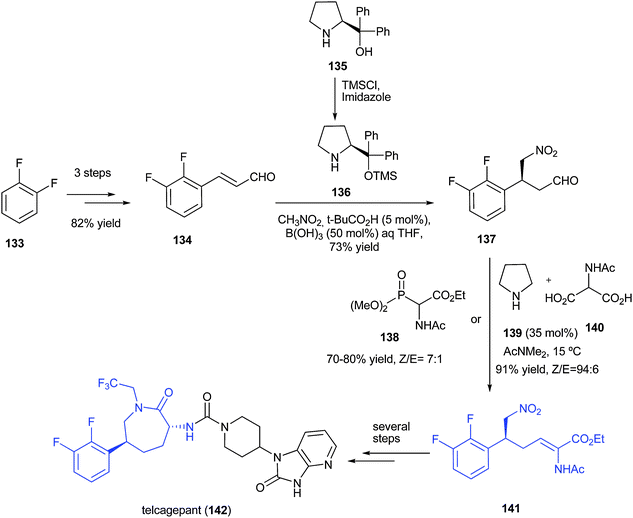
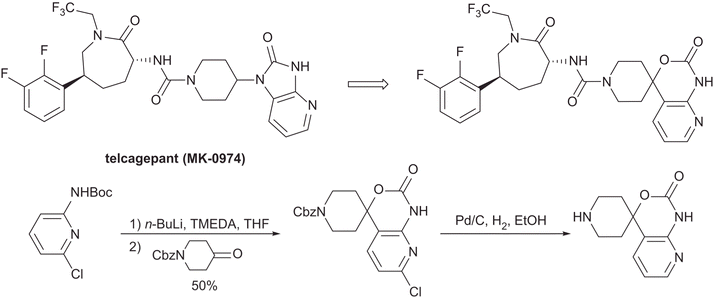
Asymmetric Synthesis of Telcagepant
http://pubs.acs.org/doi/abs/10.1021/jo101704b

References
- Jump up^ Ho, Tw; Mannix, Lk; Fan, X; Assaid, C; Furtek, C; Jones, Cj; Lines, Cr; Rapoport, Am; Mk-0974, Protocol, 004, Study, Group (Apr 2008). “Randomized controlled trial of an oral CGRP receptor antagonist, MK-0974, in acute treatment of migraine”. Neurology. 70 (16): 1304–12. doi:10.1212/01.WNL.0000286940.29755.61. PMID 17914062.
- Jump up^ Ho TW, Ferrari MD, Dodick DW, et al. (December 2008). “Efficacy and tolerability of MK-0974 (telcagepant), a new oral antagonist of calcitonin gene-related peptide receptor, compared with zolmitriptan for acute migraine: a randomised, placebo-controlled, parallel-treatment trial”. Lancet. 372 (9656): 2115–23. doi:10.1016/S0140-6736(08)61626-8. PMID 19036425.
- Jump up^ Molecule of the Month February 2009
- Jump up^ Clinical trial number NCT00797667 for “MK0974 for Migraine Prophylaxis in Patients With Episodic Migraine” at ClinicalTrials.gov
- Jump up^ Merck & Co.: Memo to all US study locations involved in protocol MK0974-049
- Jump up^ Merck Announces Second Quarter 2011 Financial Results
 |
|
 |
|
| Clinical data | |
|---|---|
| Routes of administration |
Oral |
| ATC code | none |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Biological half-life | 5–8 hours |
| Identifiers | |
| CAS Number | 781649-09-0 |
| PubChem (CID) | 11319053 |
| IUPHAR/BPS | 703 |
| ChemSpider | 9494017 |
| UNII | D42O649ALL |
| KEGG | D09391 |
| ChEMBL | CHEMBL236593 |
| Chemical and physical data | |
| Formula | C26H27F5N6O3 |
| Molar mass | 566.5283 g/mol |
| 3D model (Jmol) | Interactive image |
| Patent ID | Patent Title | Submitted Date | Granted Date |
|---|---|---|---|
| US8394767 | Methods of treating cancer using the calcitonin-gene related peptide (â??CGRPâ??) receptor antagonist CGRP8-37 | 2011-01-10 | 2013-03-12 |
| US8080544 | PRODRUGS OF CGRP RECEPTOR ANTAGONISTS | 2010-11-25 | 2011-12-20 |
| US7893052 | CGRP RECEPTOR ANTAGONISTS | 2010-11-25 | 2011-02-22 |
| US2010286122 | CGRP Antagonist Salt | 2010-11-11 | |
| US7829699 | Process for the Preparation of Cgrp Antagonist | 2009-11-12 | 2010-11-09 |
| US7772224 | CGRP RECEPTOR ANTAGONISTS | 2009-07-30 | 2010-08-10 |
| US7745427 | Cgrp Receptor Antagonists | 2008-04-17 | 2010-06-29 |
| US7718796 | Process for the preparation of Caprolactam Cgrp Antagonist | 2009-05-14 | 2010-05-18 |
| US2010009967 | SOLID DOSAGE FORMULATIONS OF TELCAGEPANT POTASSIUM | 2010-01-14 | |
| US2009176986 | Process for the Preparation of Pyridine Heterocycle Cgrp Antagonist Intermediate | 2009-07-09 |
“ALL FOR DRUGS” CATERS TO EDUCATION GLOBALLY, No commercial exploits are done or advertisements added by me. This article is a compilation for educational purposes only.
P.S. : The views expressed are my personal and in no-way suggest the views of the professional body or the company that I represent
///////////Telcagepant, MK-0974
C1CC(C(=O)N(CC1C2=C(C(=CC=C2)F)F)CC(F)(F)F)NC(=O)N3CCC(CC3)N4C5=C(NC4=O)N=CC=C5
















Sorry, the comment form is closed at this time.