Dec 212016
Cas 1204588-48-6
MF C21 H20 N4 O4
MW 392.41
Benzenamine, 2,4-dinitro-N-[(2E,4E)-4-phenyl-5-(1-pyrrolidinyl)-2,4-pentadien-1-ylidene]-, [N(E)]-
(E)-2,4-Dinitro-N-((2E,4E)-4-phenyl-5-(pyrrolidin-1-yl)penta-2,4-dienylidene)aniline
Molbank 2009, 2009(3), M604; doi:10.3390/M604
Synthesis of (E)-2,4-Dinitro-N-((2E,4E)-4-phenyl-5-(pyrrolidin-1-yl)penta-2,4-dienylidene)aniline
1Laboratory of Organic Chemistry, Faculty of Science, University of Guilan, P.O.Box 1914, Rasht, Iran,
2Departments of Physical Chemistry, Faculty of Science, University of Guilan, P.O.Box 1914, Rasht, Iran
3Departments of Analytical Chemistry, Faculty of Science, University of Guilan, P.O.Box 1914, Rasht, Iran
*Author to whom correspondence should be addressed
mahmoodi@guilan.ac.ir, m-chem41@guilan.ac.ir, aggilani@guilan.ac.ir, arvand@guilan.ac.ir, nosmahmoodi@gmail.com
Abstract:
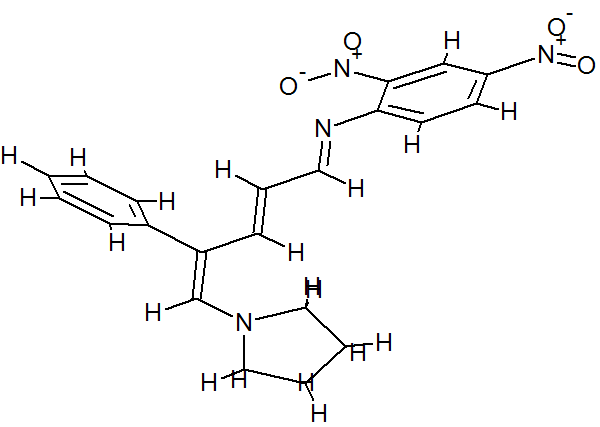
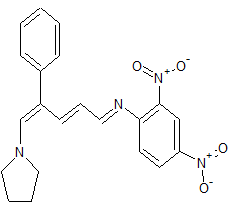
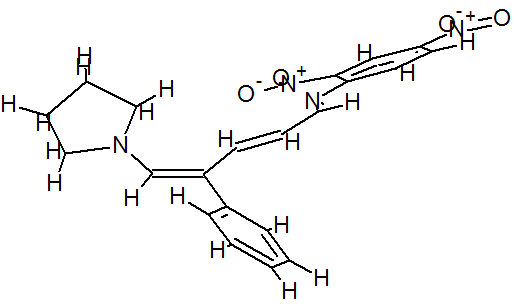
(E)-2,4-Dinitro-N-((2E,4E)-4-phenyl-5-(pyrrolidin-1-yl)penta-2,4-dienylidene) aniline dye was prepared in one pot by reaction of premade N-2,4-dinitrophenyl-3-phenylpyridinium chloride (DNPPC) and pyrrolidine in absolute MeOH.
Keywords:
N-2,4-dinitrophenyl-3-phenylpyridinium chloride (DNPPC); photochromic; pyridinium salt
N-2,4-Dinitrophenyl-3-phenylpyridinium chloride (DNPPC) 1 was prepared according to the literature method [1,2,3,4,5,6,7]. Recently, we became interested in the synthesis of photochromic compounds [8,9,10]. The UV-Vis spectra under irradiation of UV light of dye 2 indicate photochromic properties for this molecule. The salt 1 was premade and typically isolated and purified by recrystallization and characterized. To a solution of 1-chloro-2,4-dinitrobenzene (1.42 g, 7.01 mmol) in acetone (10 mL) was added 3-phenylpyridine (1.0 mL, 6.97 mmol). The reaction was heated at reflux for 48 h. The solvent was removed under reduced pressure and the red residue was stirred in hexanes. The precipitated product was collected by vacuum filtration to afford pure pyridinium salt 1 as a reddish brown solid (2.23 g, 6.25 mmol, 90%). 1H NMR (CDCl3, 500 MHz): δ (ppm) 9.9 (s, 1H), 9.4 (d, J = 6.0 Hz, 1H), 9.3 (d, J = 8.3 Hz, 1H), 9.2 (d, J = 2.2 Hz, 1H), 9.0 (dd, J = 8.7, 2.4 Hz, 1H), 8.5-8.6 (m, 2H), 8.0 (d, J = 7.3 Hz, 2H), 7.6- 7.7 (m, 3H); 13C NMR (CDCl3, 125 MHz): δ (ppm) 149.2, 145.6, 144.3, 144.2, 143.0, 139.2, 138.7, 132.5, 132.3, 130.6, 130.2, 129.6, 128.0, 127.6, 121.3; IR (KBr pellet) 3202, 3129, 2994, 2901, 1609 cm-1; m. p. = 182-183 °C; HRMS m/z Calcd for C17H12N3O4+ (M)+ 322.0828, found 322.0836.
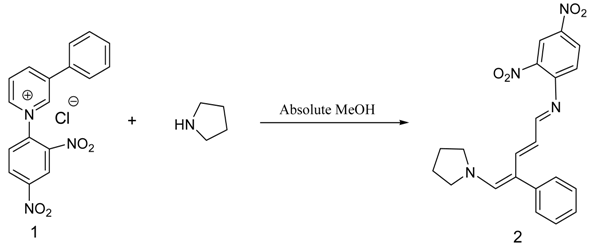
Reaction of pyrrolidine with salt (1) leads to the opening of the pyridinium ring and formation of dye 2. This dye was prepared from reaction of salt 1 (0.5 g, 1.4 mmol) in 5 mL absolute MeOH after cooling a reaction mixture to -10oC and keeping at this temperature for 15 min. To this was added pyrrolidine (0.1 g, 1.4 mmol) in 3 mL absolute MeOH over a period of 10 min. The prepared solid was filtered, washed with CH2Cl2, dried and recrystallized from n-hexane to yield 68% (0.37 g, 0.95 mmol) of pure metallic greenish-brown 2,
m.p. = 146 oC.
IR (KBr): 3040, 2950, 1616, 1514, 1492, 1469, 1321, 1215, 1170, 1105, 956, 904, 862, 727 cm-1.
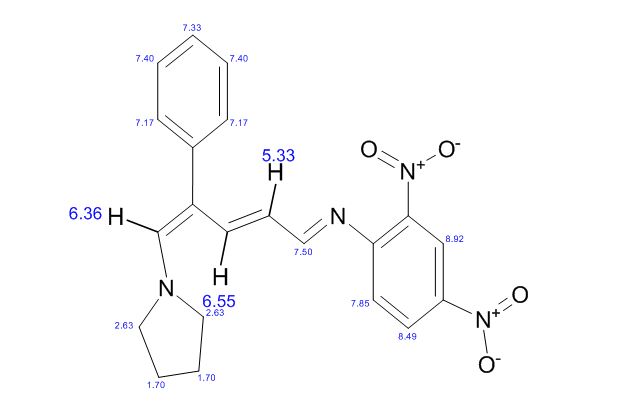
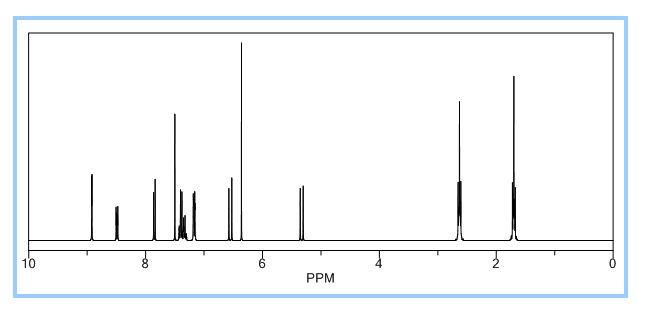
1H NMR (500 MHz, CDCl3): δ (ppm) 8.7 (d, J = 2.4 Hz, 1H) 8.3 (dd, J = 2.4, 8.84 Hz, 1H), 8.0 (s, 1H), 7.5 (d, J = 7.4 Hz, 2H), 7.4-7.5 (t, J = 7.5 Hz, 2H), 7.3-7.4 (m, 1H), 7.2 (d, J = 12.5 Hz, 1H), 7.1 (d, J = 8.9 Hz, 1H), 7.0 (d, J = 12.1 Hz, 1H), 5.4 (t, J = 12.2 Hz, 1H), 3.3 (br, 4H), 2.0 (br, 4H);
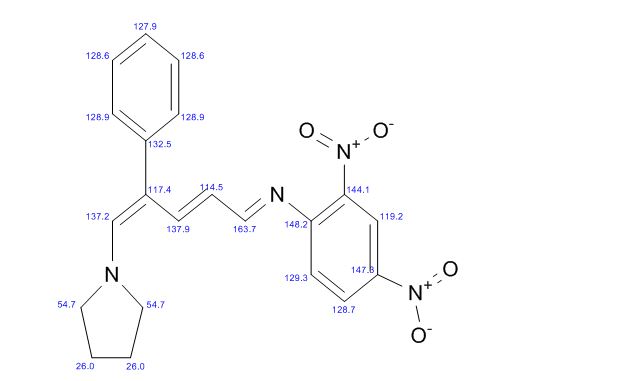
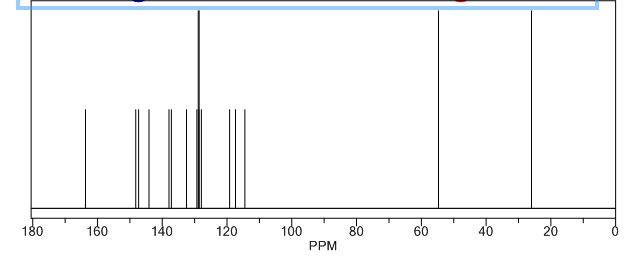
13C NMR (125 MHz, CDCl3): δ (ppm) 22.0, 55.6, 114.7, 117.4, 120.0, 124.1, 126.4, 128.7, 128,8, 129.0, 132.7, 137.1, 137.3, 142.9, 147.8, 150.2, 163.8.
Anal. Calcd for C21H20N4O4: %C = 64.28, %H = 5.14, %N = 14.28. Found: %C = 64.08, %H = 5.11, %N = 14.07.
1H NMR PREDICT
ACTUAL….
1H NMR (500 MHz, CDCl3): δ (ppm) 8.7 (d, J = 2.4 Hz, 1H) 8.3 (dd, J = 2.4, 8.84 Hz, 1H), 8.0 (s, 1H), 7.5 (d, J = 7.4 Hz, 2H), 7.4-7.5 (t, J = 7.5 Hz, 2H), 7.3-7.4 (m, 1H), 7.2 (d, J = 12.5 Hz, 1H), 7.1 (d, J = 8.9 Hz, 1H), 7.0 (d, J = 12.1 Hz, 1H), 5.4 (t, J = 12.2 Hz, 1H), 3.3 (br, 4H), 2.0 (br, 4H);
13 C NMR PREDICT
ACTUAL…….13C NMR (125 MHz, CDCl3): δ (ppm) 22.0, 55.6, 114.7, 117.4, 120.0, 124.1, 126.4, 128.7, 128,8, 129.0, 132.7, 137.1, 137.3, 142.9, 147.8, 150.2, 163.8.
////////////Synthesis, (E)-2,4-Dinitro-N-((2E,4E)-4-phenyl-5-(pyrrolidin-1-yl)penta-2,4-dienylidene)aniline
[O-][N+](=O)c3ccc(\N=C\C=C\C(=C/N1CCCC1)c2ccccc2)c([N+]([O-])=O)c3













Sorry, the comment form is closed at this time.