

Characterization Data of Compound 7
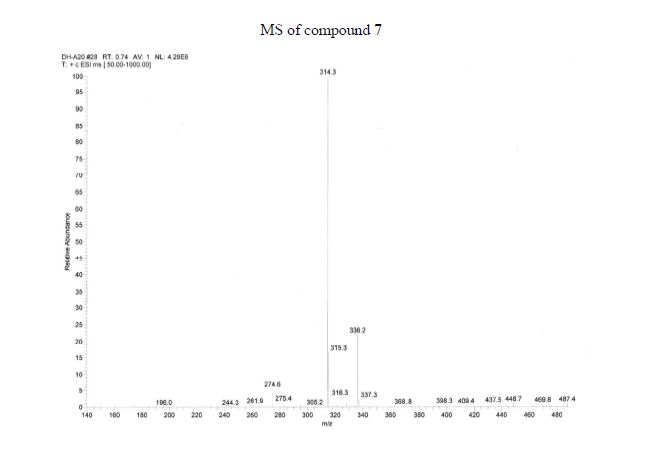
Mp: 118–120 °C. MS (M + H+): 314.
HRMS (ESI) m/z: Calcd for C16H15N3NaO4, (M + Na+): 336.0960. Found: 336.0899.
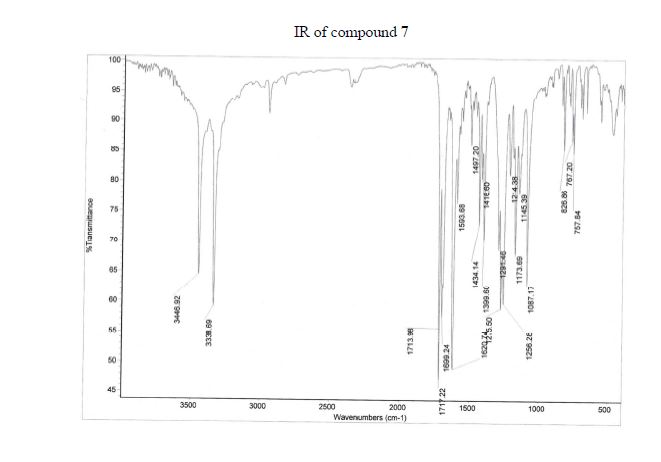
IR (KBr) ν/cm–1: 3447, 3339, 1717, 1714, 1699, 1594.
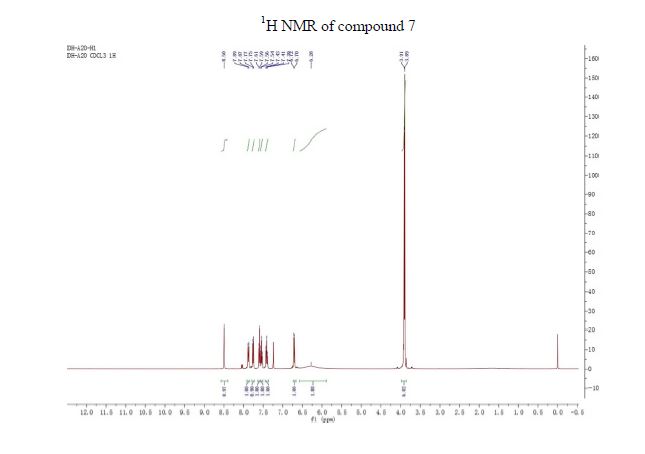
1H NMR (CDCl3, 400 MHz) δ/ppm: 8.50 (s, 1H, Ar–H), 7.88 (d, J = 8.8 Hz, 1H, Ar–H), 7.76 (d, J = 7.6 Hz, 1H, Ar–H), 7.60 (d, J = 8.0 Hz, 1H, Ar–H), 7.54 (t, J = 7.2 Hz, 1H, Ar–H), 7.41 (t, J = 7.2 Hz, 1H, Ar–H), 6.71 (d, J = 9.2 Hz, 1H, Ar–H), 6.28 (br s, 2H, −NH2), 3.91 (s, 3H, −CH3), 3.89 (s, 3H, −CH3).
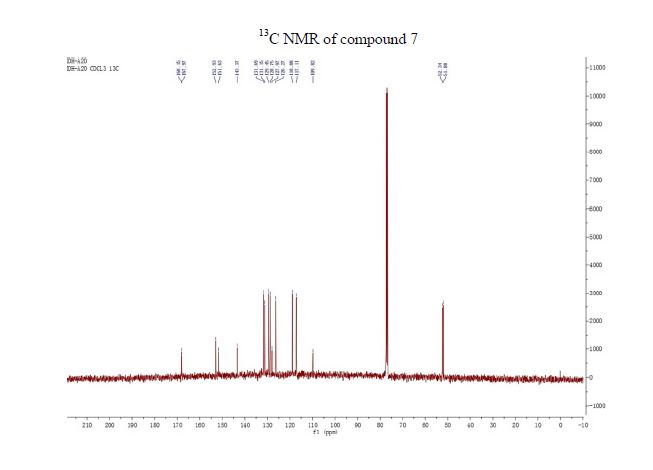
13C NMR (CDCl3, 100 MHz) δ/ppm: 168.2, 168.0, 152.9, 151.6, 143.4, 131.7, 131.2, 129.4, 128.8, 128.0, 126.3, 118.9, 117.1, 109.8, 52.3, 51.9.
Continuous-Flow Diazotization for Efficient Synthesis of Methyl 2-(Chlorosulfonyl)benzoate: An Example of Inhibiting Parallel Side Reactions
† National Engineering Research Center for Process Development of Active Pharmaceutical Ingredients, Collaborative Innovation Center of Yangtze River Delta Region Green Pharmaceuticals, Zhejiang University of Technology, Hangzhou 310014, P. R. China
‡ Key Laboratory for Green Pharmaceutical Technologies and Related Equipment of Ministry of Education, College of Pharmaceutical Sciences, Zhejiang University of Technology, Hangzhou 310014, P. R. China
Org. Process Res. Dev., Article ASAP
DOI: 10.1021/acs.oprd.6b00238
Publication Date (Web): November 17, 2016
Copyright © 2016 American Chemical Society
Abstract
An expeditious process for the highly efficient synthesis of methyl 2-(chlorosulfonyl)benzoate was described, which involved the continuous-flow diazotization of methyl 2-aminobenzoate in a three-inlet flow reactor via a cross joint followed by chlorosulfonylation in the tandem tank reactor. The side reaction such as hydrolysis was decreased eminently from this continuous-flow process even at a high concentration of hydrochloric acid. The mass flow rate of methyl 2-aminobenzoate was 4.58 kg/h, corresponding to an 18.45 kg/h throughput of diazonium salt solution. The potential of inhibiting parallel side reactions by conducting in a flow reactor was successfully demonstrated in this method.





















Sorry, the comment form is closed at this time.