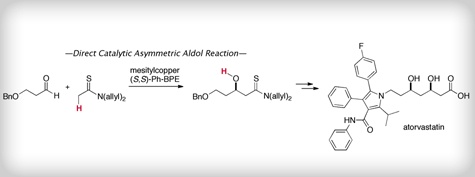
“A simplified catalytic system for direct catalytic asymmetric aldol reaction of thioamides; application to an enantioselective synthesis of atorvastatin”
Kawato, Y.; Iwata, M.; Yazaki, R.; Kumagai, N.; Shibasaki, M.
Tetrahedron 2011, 67, 6539.
A simplified catalytic system for direct catalytic asymmetric aldol reaction of thioamides; application to an enantioselective synthesis of atorvastatin
- a Institute of Microbial Chemistry, Tokyo, 3-14-23 Kamiosaki, Shinagawa-ku, Tokyo 141-0021, Japan
- b Graduate School of Pharmaceutical Sciences, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-0033, Japan
http://www.sciencedirect.com/science/article/pii/S004040201100799X
Corresponding authors. Tel.: þ81 3 3447 7779; fax: þ81 3 3441 7589 (M.S.); tel.: þ81 3 3441 8133; fax: þ81 3 3441 7589 (N.K.); e-mail addresses: nkumagai@bikaken.or.jp (N. Kumagai), mshibasa@bikaken.or.jp (M. Shibasaki).
atorvastatin as a colorless solid. (54.8 mg, 67% over three steps). Colorless solid;
IR (KBr) n 3410, 2964, 2929, 1731, 1652, 1529, 1508, 1438, 1315, 1241, 1226 cm1 ;
1 H NMR (CD3OD) d 7.30e7.29 (m, 2H), 7.25e7.20 (m, 4H), 7.15e7.13 (m, 2H), 7.11e7.02 (m, 6H), 4.08 (ddd, J¼5.3, 7.8, 16.0 Hz 1H), 4.02e3.98 (m, 1H), 3.91 (ddd, J¼5.3, 7.6, 16.0 Hz, 1H), 3.69e3.63 (m, 1H), 3.40e3.34 (m, 1H), 2.41 (dd, J¼5.2,15.5 Hz,1H), 2.35 (dd, J¼7.6,15.5 Hz,1H),1.75e1.6 (m, 2H), 1.56e1.51 (m, 1H), 1.49 (d, J¼7.1 Hz, 3H), 1.48 (d, J¼7.1 Hz, 3H), 1.47e1.43 (m, 1H);
13C NMR (CD3OD) d 175.9, 169.5, 163.8 ( 1 JCF¼245.5 Hz), 139.9, 139.1, 139.1, 136.4, 134.7 (3 JCF¼7.2 Hz), 131.0, 130.3 (4 JCF¼2.9 Hz),129.6,128.9,126.9,125.2,123.3,121.5,118.1,116.3 ( 2 JCF¼21.6 Hz), 68.6, 67.9, 44.2, 43.3, 42.2, 40.1, 27.7, 22.9, 22.8;
19F NMR (CDCl3) d 113.8; [a]D 23 þ5 (c 0.94, CH3OH);
ESI-MS m/z 581.2 [MþNa]þ; HRMS (ESI) Anal. Calcd for C33FH35N2NaO5 m/z 581.2422 [MþNa]þ, found; 581.2421.
///////













Sorry, the comment form is closed at this time.