
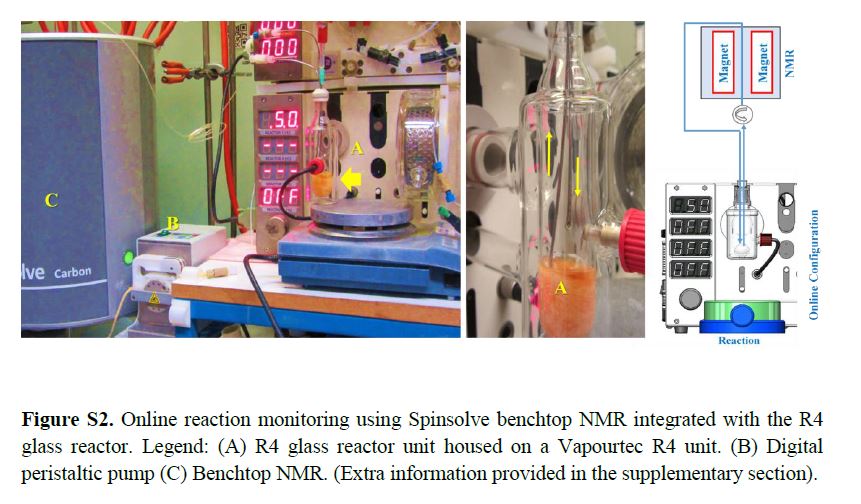
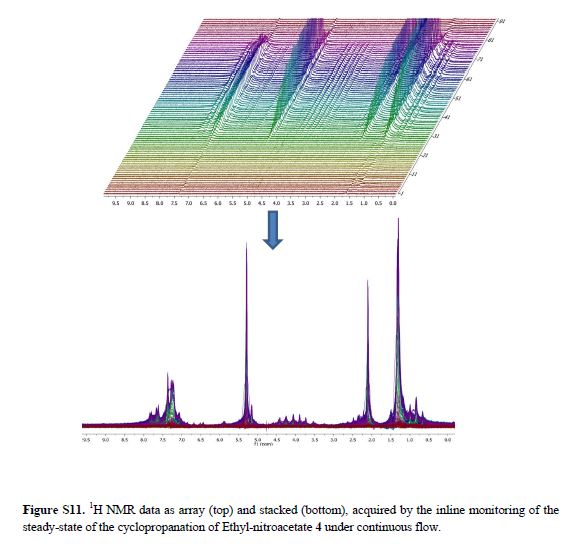
Real-time NMR spectroscopy has proven to be a rapid and an effective monitoring tool to study the hypervalent iodine(III) mediated cyclopropanation. With the ever increasing number of new synthetic methods for carbon–carbon bond formation, the NMR in situ monitoring of reactions is becoming a highly desirable enabling method. In this study, we have demonstrated the versatility of benchtop NMR using inline and online real-time monitoring methods to access mutually complementary information for process understanding, and we developed new approaches for real-time monitoring addressing challenges associated with better integration into continuous processes.
Continuous Processing and Efficient in Situ Reaction Monitoring of a Hypervalent Iodine(III) Mediated Cyclopropanation Using Benchtop NMR Spectroscopy
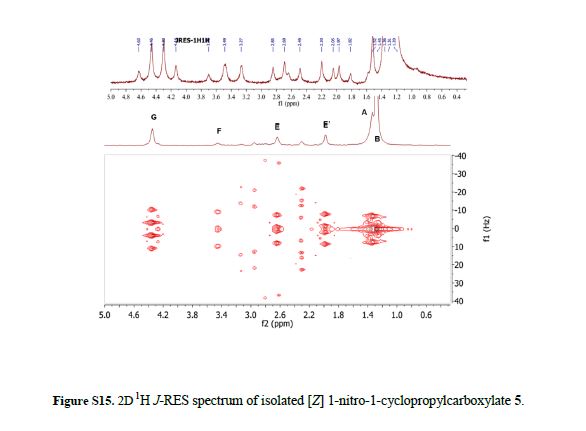
Ethyl 2-(4-tert-butylphenyl)-1-nitrocyclopropanecarboxylate (5):
[E]-isomer: 1H NMR (600 MHz, CDCl3): δ 0.80-0.85 (t, J = 7.1 Hz, 3H), 1.29 (s, 9H), 2.16-2.21 (dd, J = 10.7, 6.6 Hz, 1H), 2.41-2.46 (dd, J = 9.1, 6.6 Hz, 1H), 3.72-3.77 (m, 1H), 3.88-4.04 (m, 2H), 7.12-7.15 (d, J = 8.3 Hz, 2H), 7.30-7.37 (d, J = 8.4 Hz, 2H).
13C NMR (150 MHz, CDCl3) δ 161.96, 151.38, 128.96, 128.15, 125.37, 71.71, 62.37, 34.54, 33.91, 31.21, 20.73, 13.35.
HRMS (ESI) Calcd. for C16H21NO4 ([M+H]+): 292.15, Found 292.15:
[Z]-isomer: 1H NMR (600 MHz, CDCl3): δ 1.30 (s, 9H), 1.34-1.37 (t, J = 7.1 Hz, 3H), 2.00-2.04 (dd, J = 9.9, 6.9 Hz, 1H), 2.64-2.68 (dd, J = 9.2, 6.9 Hz, 1H), 3.43-3.48 (t, J = 9.6 Hz, 1H), 4.31-4.41 (m, 2H), 7.14-7.17 (d, J = 8.3 Hz, 2H), 7.32-7.36 (d, J = 8.4 Hz, 2H).
13C NMR (150 MHz, CDCl3) δ 165.40, 151.56, 128.33, 127.99, 125.63, 72.63, 63.14, 34.55, 33.48, 31.22, 20.08, 13.98.
Zhu, S.; Perman, J. A.; Zhang, X. P. Angew. Chem. Int. Ed. 2008, 47, 8460-8463.

/////////














Sorry, the comment form is closed at this time.