Ring-locking enables selective anhydrosugar synthesis from carbohydrate pyrolysis
DOI: 10.1039/C6GC01600F, Paper
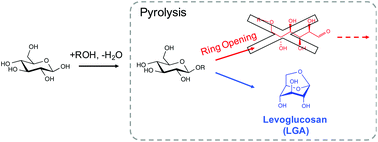
The nonselective nature of glucose pyrolysis chemistry can be controlled by preventing the sugar ring from opening and fragmenting.
Ring-locking enables selective anhydrosugar synthesis from carbohydrate pyrolysis
E-mail: mswong@rice.edu
E-mail: zczhang@dicp.ac.cn
DOI: 10.1039/C6GC01600F
The selective production of platform chemicals from thermal conversion of biomass-derived carbohydrates is challenging. As precursors to natural products and drug molecules, anhydrosugars are difficult to synthesize from simple carbohydrates in large quantities without side products, due to various competing pathways during pyrolysis. Here we demonstrate that the nonselective chemistry of carbohydrate pyrolysis is substantially improved by alkoxy or phenoxy substitution at the anomeric carbon of glucose prior to thermal treatment. Through this ring-locking step, we found that the selectivity to 1,6-anhydro-β-D-glucopyranose (levoglucosan, LGA) increased from 2% to greater than 90% after fast pyrolysis of the resulting sugar at 600 °C. DFT analysis indicated that LGA formation becomes the dominant reaction pathway when the substituent group inhibits the pyranose ring from opening and fragmenting into non-anhydrosugar products. LGA forms selectively when the activation barrier for ring-opening is significantly increased over that for 1,6-elimination, with both barriers affected by the substituent type and anomeric position. These findings introduce the ring-locking concept to sugar pyrolysis chemistry and suggest a chemical-thermal treatment approach for upgrading simple and complex carbohydrates.
////////Ring-locking , selective anhydrosugar, carbohydrate pyrolysis, synthesis













Sorry, the comment form is closed at this time.