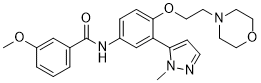
Temanogrel
APD 791
| TEMANOGREL; APD791; CHEMBL1084617; UNII-F42Z27575A; 887936-68-7; 3-Methoxy-N-[3-(2-methyl-2H-pyrazol-3-yl)-4-(2-morpholin-4-yl-ethoxy)-phenyl]-benzamide; | |
| Molecular Formula: | C24H28N4O4 |
|---|---|
| Molecular Weight: | 436.50352 g/mol |
- Originator Arena Pharmaceuticals
- Developer Arena Pharmaceuticals; Ildong Pharmaceutical
- Class Antithrombotics; Small molecules
- Mechanism of Action Serotonin 2A receptor inverse agonists
Phase I Arterial thrombosis 
Most Recent Events
- 30 Mar 2016 Arena Pharmaceuticals has patents pending for Temanogrel in 12 regions, including Brazil (Arena Pharmaceuticals 10-K; march 2016)
- 30 Mar 2016 Arena Pharmaceuticals has patent protection for Temanogrel in 87 regions, including USA, Japan, China, Germany, France, Italy, the United Kingdom, Spain, Canada, Russia, India, Australia and South Korea
- 01 Mar 2015 Ildong Pharmaceutical initiates enrolment in a phase I trial for Arterial thrombosis in South Korea (NCT02419820)
A 5-HT2A inverse agonist potentially for the reduction of the risk of arterial thrombosis.

APD-791
CAS No. 887936-68-7
Temanogrel hydrochloride
- Molecular FormulaC24H29ClN4O4
- Average mass472.965
Temanogrel, also known as APD791, is a highly selective 5-hydroxytryptamine2A receptor inverse agonist under development for the treatment of arterial thrombosis. APD791 displayed high-affinity binding to membranes (K(i) = 4.9 nM) and functional inverse agonism of inositol phosphate accumulation (IC(50) = 5.2 nM) in human embryonic kidney cells stably expressing the human 5-HT(2A) receptor. APD791 was greater than 2000-fold selective for the 5-HT(2A) receptor versus 5-HT(2C) and 5-HT(2B) receptors. APD791 inhibited 5-HT-mediated amplification of ADP-stimulated human and dog platelet aggregation (IC(50) = 8.7 and 23.1 nM, respectively)
Arterial thrombosis is the formation of a blood clot or thrombus inside an artery or arteriole that restricts or blocks the flow of blood and, depending upon location, can result in acute coronary syndrome or stroke. The formation of a thrombus is usually initiated by blood vessel injury, which triggers platelet aggregation and adhesion of platelets to the vessel wall. Treatments aimed at inhibiting platelet aggregation have demonstrated clear clinical benefits in the setting of acute coronary syndrome and stroke. Current antiplatelet therapies include aspirin, which irreversibly inhibits cyclooxygenase (COXa
Abbreviations: COX, cyclooxygenase; ADP, adenosine diphosphate; SAR, structure−activity relationship; hERG, human ether-a-go-go-related gene; CNS, central nervous system; 5-HT, serotonin; AUC, area under the plasma concentration time curve, iv, intravenous; IP, inositol phosphate.
) and results in reduced thromboxane production, clopidogrel and prasugrel, which inhibit platelet adenosine diphosphate (ADP) P2Y12 receptors, and platelet glycoprotein IIb/IIIa receptor antagonists. Another class of antiplatelet drugs, protease-activated thrombin receptor (PAR-1) antagonists, are also being evaluated in the clinic for the treatment of acute coronary syndrome. The most advanced candidate in this class, N-[(1R,3aR,4aR,6R,8aR,9S,9aS)-9-{2-[5-(3-fluorophenyl)pyridin-2-yl]vinyl}-1-methyl-3-oxoperhydro-naphtho[2,3-c]furan-6-yl]-carbamic acid ethyl ester (SCH-530348), is currently in phase 3 trials for the prevention of arterial thrombosis.

Figure 1. Serotonin and known 5-HT2A receptor antagonists.
SYNTHESIS
PAPER
Journal of Medicinal Chemistry (2010), 53(11), 4412-4421.
http://pubs.acs.org/doi/abs/10.1021/jm100044a

Serotonin, which is stored in platelets and is released during thrombosis, activates platelets via the 5-HT2A receptor. 5-HT2A receptor inverse agonists thus represent a potential new class of antithrombotic agents. Our medicinal program began with known compounds that displayed binding affinity for the recombinant 5-HT2A receptor, but which had poor activity when tested in human plasma platelet inhibition assays. We herein describe a series of phenyl pyrazole inverse agonists optimized for selectivity, aqueous solubility, antiplatelet activity, low hERG activity, and good pharmacokinetic properties, resulting in the discovery of 10k (APD791). 10k inhibited serotonin-amplified human platelet aggregation with an IC50 = 8.7 nM and had negligible binding affinity for the closely related 5-HT2B and 5-HT2C receptors. 10k was orally bioavailable in rats, dogs, and monkeys and had an acceptable safety profile. As a result, 10k was selected further evaluation and advanced into clinical development as a potential treatment for arterial
Discovery and Structure−Activity Relationship of 3-Methoxy-N-(3-(1-methyl-1H-pyrazol-5-yl)-4-(2-morpholinoethoxy)phenyl)benzamide (APD791): A Highly Selective 5-Hydroxytryptamine2A Receptor Inverse Agonist for the Treatment of Arterial Thrombosis
Additional Information
Oral administration of APD791 to dogs resulted in acute (1-h) and subchronic (10-day) inhibition of 5-HT-mediated amplification of collagen-stimulated platelet aggregation in whole blood. Two active metabolites, APD791-M1 and APD791-M2, were generated upon incubation of APD791 with human liver microsomes and were also indentified in dogs after oral administration of APD791. The affinity and selectivity profiles of both metabolites were similar to APD791. These results demonstrate that APD791 is an orally available, high-affinity 5-HT(2A) receptor antagonist with potent activity on platelets and vascular smooth muscle.(http://www.ncbi.nlm.nih.gov/pubmed/19628629).
PATENT
WO 2006055734
https://google.com/patents/WO2006055734A2?cl=en
Example 1.88: Preparation of 3-methoxy-N-[3-(2-methyl-2H-pyrazol-3-yl)-4-(2-morpholin~
4-yl-ethoxy)-phenyl]-benzamide (Compound 733).
A mixture of 3-(2-methyl-2H-pyrazol-3-yl)-4-(2-morpholin-4-yl-ethoxy)-phenylamine (120 mg, 0.40 mmole), 3-methoxy-benzoyl chloride (81 mg, 0.48 mmole), and triethylamine (0.1 mL, 0.79 mmole) in 5 mL THF was stirred at room temperature for 10 minutes. The mixture was purified by HPLC to give the title compound as a white solid (TFA salt, 88 mg, 51 %). 1H NMR ( Acetone-^, 400 MHz) 2.99-3.21 (m, 2H), 3.22-3.45 (m, 2H), 3.66 (t, J= 4.80 Hz, 2H), 3.75 (s, 3H), 3.85 (s, 3H), 3.79-3.89 (m, 4H), 4.58 (t, J= 4.80 Hz, 2H), 6.29 (d, J= 2.02 Hz IH), 7.13 (dd, J= 8.34, 2.53 Hz, IH), 7.22 (d, J= 8.84 Hz, IH), 7.42 (t, J= 7.83 Hz, IH), 7.47 (d, J= 1.77 Hz, IH), 7.52 (t, J= 1.77 Hz, IH), 7.56 (d, J= 7.07 Hz, IH), 7.80-7.83 (m, IH), 7.91-7.96 (m, IH), 9.54 (s, NH). Exact mass calculated for C24H28N4O4 436.2, found 437.5 (MH+).
References
1: Xiong Y, Teegarden BR, Choi JS, Strah-Pleynet S, Decaire M, Jayakumar H, Dosa
PI, Casper MD, Pham L, Feichtinger K, Ullman B, Adams J, Yuskin D, Frazer J,
Morgan M, Sadeque A, Chen W, Webb RR, Connolly DT, Semple G, Al-Shamma H.
Discovery and structure-activity relationship of
3-methoxy-N-(3-(1-methyl-1H-pyrazol-5-yl)-4-(2-morpholinoethoxy)phenyl)benzamide
(APD791): a highly selective 5-hydroxytryptamine2A receptor inverse agonist for
the treatment of arterial thrombosis. J Med Chem. 2010 Jun 10;53(11):4412-21.
doi: 10.1021/jm100044a. PubMed PMID: 20455563.
2: Przyklenk K, Frelinger AL 3rd, Linden MD, Whittaker P, Li Y, Barnard MR, Adams
J, Morgan M, Al-Shamma H, Michelson AD. Targeted inhibition of the serotonin
5HT2A receptor improves coronary patency in an in vivo model of recurrent
thrombosis. J Thromb Haemost. 2010 Feb;8(2):331-40. doi:
10.1111/j.1538-7836.2009.03693.x. Epub 2009 Nov 17. PubMed PMID: 19922435; PubMed
Central PMCID: PMC2916638.
3: Adams JW, Ramirez J, Shi Y, Thomsen W, Frazer J, Morgan M, Edwards JE, Chen W,
Teegarden BR, Xiong Y, Al-Shamma H, Behan DP, Connolly DT. APD791,
3-methoxy-n-(3-(1-methyl-1h-pyrazol-5-yl)-4-(2-morpholinoethoxy)phenyl)benzamide,
a novel 5-hydroxytryptamine 2A receptor antagonist: pharmacological profile,
pharmacokinetics, platelet activity and vascular biology. J Pharmacol Exp Ther.
2009 Oct;331(1):96-103. doi: 10.1124/jpet.109.153189. Epub 2009 Jul 23. PubMed
PMID: 19628629.
| Patent ID | Date | Patent Title |
|---|---|---|
| US2015361031 | 2015-12-17 | STAT3 INHIBITOR |
| US8785441 | 2014-07-22 | 3-phenyl-pyrazole derivatives as modulators of the 5-HT2A serotonin receptor useful for the treatment of disorders related thereto |
| US2013296321 | 2013-11-07 | CRYSTALLINE FORMS AND PROCESSES FOR THE PREPARATION OF PHENYL-PYRAZOLES USEFUL AS MODULATORS OF THE 5-HT2A SEROTONIN RECEPTOR |
| US2012252813 | 2012-10-04 | CRYSTALLINE FORMS OF CERTAIN 3-PHENYL-PYRAZOLE DERIVATIVES AS MODULATORS OF THE 5-HT2A SEROTONIN RECEPTOR USEFUL FOR THE TREATMENT OF DISORDERS RELATED THERETO |
| US8148417 | 2012-04-03 | PRIMARY AMINES AND DERIVATIVES THEREOF AS MODULATORS OF THE 5-HT2A SEROTONIN RECEPTOR USEFUL FOR THE TREATMENT OF DISORDERS RELATED THERETO |
| US8148418 | 2012-04-03 | ETHERS, SECONDARY AMINES AND DERIVATIVES THEREOF AS MODULATORS OF THE 5-HT2A SEROTONIN RECEPTOR USEFUL FOR THE TREATMENT OF DISORDERS RELATED THERETO |
| US2011105456 | 2011-05-05 | 3-PHENYL-PYRAZOLE DERIVATIVES AS MODULATORS OF THE 5-HT2A SEROTONIN RECEPTOR USEFUL FOR THE TREATMENT OF DISORDERS RELATED THERETO |
| US7884101 | 2011-02-08 | 3-Phenyl-pyrazole derivatives as modulators of the 5-HT2a serotonin receptor useful for the treatment of disorders related thereto |
| US2010234380 | 2010-09-16 | CRYSTALLINE FORMS AND PROCESSES FOR THE PREPARATION OF PHENYL-PYRAZOLES USEFUL AS MODULATORS OF THE 5-HT2A SEROTONIN RECEPTOR |
| US2007244086 | 2007-10-18 | 3-Phenyl-Pyrazole Derivatives as Modulators of the 5-Ht2A Serotonin Receptor Useful for the Treatment of Disorders Related Thereto |
///////////APD-791 , 887936-68-7, Temanogrel , PHASE 1, ARENA,
CN1C(=CC=N1)C2=C(C=CC(=C2)NC(=O)C3=CC(=CC=C3)OC)OCCN4CCOCC4
C(=O)














Sorry, the comment form is closed at this time.