
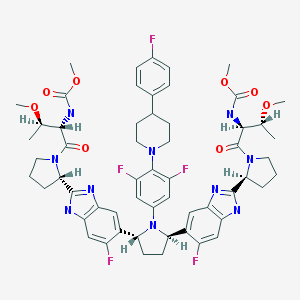
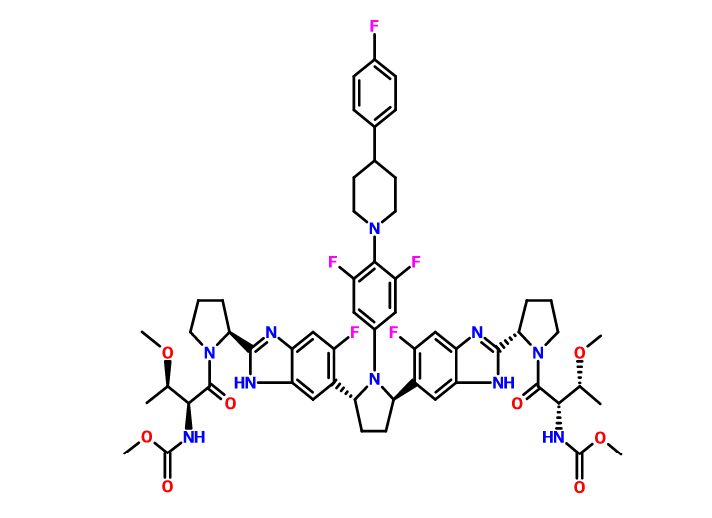
Pibrentasvir
ABT-530, Pibrentasvir, A 1325912.0
Dimethyl N,N’-([(2R,5R)-1-{3,5-difluoro-4-[4-(4-fluorophenyl)piperidin-1-yl]phenyl}pyrrolidine-2,5-diyl]bis{(6-fluoro-1H-benzimidazole-5,2-diyl)[(2S)-pyrrolidine-2,1-diyl][(2S,3R)-3-methoxy-1-oxobutane-1,2-diyl]})biscarbamate
Methyl {(2S,3R)-1-[(2S)-2-{5-[(2R,5R)-1-{3,5-difluoro-4-[4-(4-fluorophenyl)piperidin-1-yl]phenyl}-5-(6-fluoro-2-{(2S)-1-[N-(methoxycarbonyl)-O-methyl-L-threonyl]pyrrolidin-2-yl}-1H-benzimidazol-5-yl)pyrrolidin-2-yl]-6-fluoro-1H-benzimidazol-2-yl}pyrrolidin-1-yl]-3-methoxy-1-oxobutan-2-yl}carbamate
Dimethyl N,N’-(((2R,5R)-1-(3,5-difluoro-4-(4-(4-fluorophenyl)piperidin-1-yl)phenyl)pyrrolidine-2,5-diyl)bis((6-fluoro-1H-benzimidazole-5,2-diyl)((2S)-pyrrolidine-2,1-diyl)((2S,3R)-3-methoxy-1-oxobutane-1,2-diyl)))biscarbamate
Methyl ((2S,3R)-1-((2S)-2-(5-((2R,5R)-1-(3,5-difluoro-4-(4-(4-fluorophenyl)piperidin-1-yl)phenyl)-5-(6-fluoro-2-((2S)-1-(N-(methoxycarbonyl)-O-methyl-L-threonyl)pyrrolidin-2-yl)-1H-benzimidazol-5-yl)pyrrolidin-2-yl)-6-fluoro-1H-benzimidazol-2-yl)pyrrolidin-1-yl)-3-methoxy-1-oxobutan-2-yl)carbamate
Phase III
Abbott Laboratories INNOVATOR
A protease inhibitor potentially for the treatment of HCV infection.
Hepatitis C virus NS 5 protein inhibitors
![]()
CAS No. 1353900-92-1
| MF | C57H65F5N10O8 |
|---|
MW 1113.1925 MW
Pibrentasvir
- CLINICAL https://clinicaltrials.gov/search/intervention=A-1325912.0%20OR%20ABT-530%20OR%20Pibrentasvir
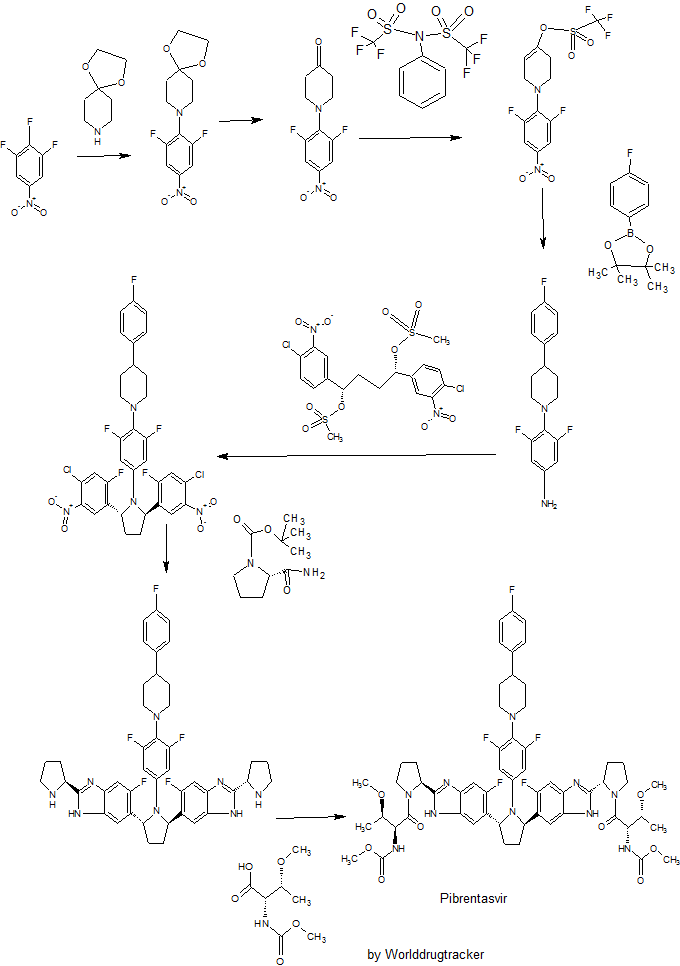
SYNTHESIS
PATENT
WO 2012051361
http://www.google.com/patents/WO2012051361A1?cl=en
Example 3.52 methyl {(2S,3R)-l-[(2S)-2-{5-[(2R,5R)-l-{3,5-difluoro-4-[4-(4- fluorophenyl)piperidin-l-yl]phenyl}-5-(6-fluoro-2-{(2.S)-l-[A^-(methoxycarbonyl)-0-methyl-L- threonyl]pyiTolidin-2-yl}-l f-benzimidazol-5-yl)pyiTolidin-2-yl]-6-fluoro-l f-benzimidaz yl}pyrrolidin-l-yl]-3-methoxy-l-oxobutan-2-yl}carbamatelH NMR (400 MHz, DMSO) δ 12.36 – 12.06 (m, 2H), 7.41 (dd, J = 11.2, 6.3, 1H), 7.34 (dd, J = 10.4, 4.8, 1H), 7.30 – 7.20 (m, 3H), 7.17 – 6.98 (m, 5H), 5.98 – 5.82 (m, 2H), 5.65 – 5.47 (m, 2H), 5.17 – 5.06 (m, 2H), 4.25 (dd, J = 15.6, 8.1, 2H), 3.88 – 3.74 (m, 3H), 3.53 (d, J = 1.3, 6H), 3.49 – 3.38 (m, 2H), 3.31 (d, 1H), 3.25 (d, J = 3.7, 1H), 3.13 (d, J = 1.3, 3H), 3.03 (d, J = 2.3, 3H), 3.00 – 2.84 (m, 3H), 2.60 – 2.53 (m, J = 2.5, 2H), 2.26 – 1.55 (m, 14H), 1.28 – 1.13 (m, 1H), 1.10 – 0.88 (m, 6H). MS (ESI; M+H) m/z = 1113.4.
Intermediate 5
( 15,45)- 1 ,4-bis(4-chloro-3 -nitrophenyl)butane- 1 ,4-diyl dimethanesulfonate Intermediate 5A
2-bromo- 1 -(4-chloro-3 -nitrophenyl)ethanone
Method A:
To a flask equipped with a magnetic stir bar and under an atmosphere of N2 was added 4′- chloro-3 ‘-nitroacetophenone (10.0 g, 50.1 mmol) and THF (100 mL). To this stirring mixture was added portion-wise phenyltrimethylammonium tribromide (19.78 g, 52.6 mmol) over a 15 minutes time period. The resultant mixture was then stirred with monitoring every hour via LCMS. After 3 hours, the mixture was then filtered and resulting solids washed with EtOAc. The organic solution was then concentrated, and 10% aq. NaHCC^ were added, and the mixture was washed with EtOAc (2×300 mL). The combined organic layers were then washed with brine, dried (MgSO^), filtered and concentrated. The residue material was then subjected to purification via crystallization. The residue was dissolved in EtOAc (100 mL) and hexanes were slowly added until the mixture was cloudy. After standing for a few hours, 2-bromo- l-(4-chloro-3-nitrophenyl)ethanone (9.81 g, 70%) was collected as an off white colored solid product. !H NMR (500 MHz, DMSO-cfe) δ ppm 5.00 (s, 2 H) 7.98 (d, J=8.54 Hz, 1 H) 8.24 (dd, J=8.54, 2.14 Hz, 1 H) 8.61 (d, J=1.98 Hz, 1 H).
Method B:
In a 500 mL round-bottomed flask was added l -(4-chloro-3-nitrophenyl)ethanone (1 1.98 g, 60 mmol) in benzene (75 mL) to give a white suspension. Bromine (9.59 g, 60.0 mmol) was added dropwise over 5 minutes to give a deep red solution. The mixture was stirred for 1 hour to give a yellow solution that was concentrated in vacuo to a yellow solid. Recrystallized from 9: 1 hexane/ethyl acetate gave 2-bromo-l -(4-chloro-3-nitrophenyl)ethanone as yellow needles.
Intermediate 21
(1 S,4S)-1 -(4-chloro-2-fluoro-5 -nitrophenyl)-4-(4-chloro-3 -nitrophenyl)butane- 1 ,4-diyl
dimethanesulfonate
Intermediate 21 can be made from Intermediate 20B and l-(4-chloro-3-nitrophenyl)ethanone (commercially available from Aldrich) following the general methods to prepare Intermediate 20E.
l-(2,6-difluoro-4-nitrophenyl)piperidin-4-one
The crude 8-(2,6-difluoro-4-nitrophenyl)-l,4-dioxa-8-azaspiro[4.5]decane from the preceding procedure was dissolved in 4:1 acetone:water (100 mL). Concentrated HC1 (5 mL) was added, and the resulting mixture was stirred at 50 °C for 8 hours and then cooled to room temperature. The mixture was concentrated in vacuo to approximately 20 mL, which was carefully added to concentrated aq. NaHCC^ (100 mL) and extracted with EtOAc (2 x 100 mL). The combined organic extracts were dried over Na2S04, filtered and concentrated in vacuo. The crude product was triturated with Et20 and hexanes to give a bright-yellow solid that was collected and dried to provide the title compound (7.13 g, 81%).
PATENT
The present invention features crystalline polymorphs of methyl {(2S,3R)-1- [(2S)-2-{5-[(2R,5R)-l-{3,5-difluoro-4 4-(4-fluorophenyl)piperidin-l-yl]phenyl}-5-(6-fluoro-2-{(2S)- 1 -[N-(methoxycarbonyl)-0-methyl-L-threonyl]pyrrolidin-2-yl} – 1 H-benzimidazol-5-yl)pyrrolidin- -yl] -6-fluoro- 1 H-benzimidazol-2-yl} pyrrolidin- 1 -yl] -3 -methoxy- 1 -oxobutan-2-
yl} carbamate 
, herein “Compound I”). Compound I is a potent HCV NS5A inhibitor and is described in U.S. Patent Application Publication No. 2012/0004196, which is incorporated herein by reference in its entirety.
//////////1353900-92-1, PHASE 3, ABT-530, Pibrentasvir, ABT 530, A 1325912.0
C[C@H]([C@@H](C(=O)N1CCC[C@H]1c2[nH]c3cc(c(cc3n2)[C@H]4CC[C@@H](N4c5cc(c(c(c5)F)N6CCC(CC6)c7ccc(cc7)F)F)c8cc9c(cc8F)[nH]c(n9)[C@@H]1CCCN1C(=O)[C@H]([C@@H](C)OC)NC(=O)OC)F)NC(=O)OC)OC
C[C@H]([C@@H](C(=O)

















Sorry, the comment form is closed at this time.