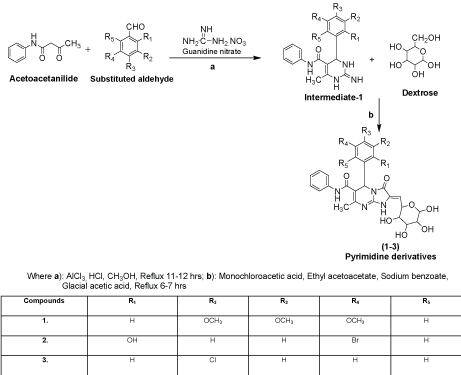
Procedure for the synthesized derivatives (1-3)
Step 1: The synthesis of Intermediate-1 (2-imino-6-methyl-4- (substituted aldehyde)-N-phenyl-1,2,3,4-tetrahydropyrimidine-5- carboxamide): A mixture of substituted aldehyde (0.01 mol), guanidine nitrate (0.015 mol) and acetoacetanilide (0.01 mol) were placed in round bottom flask in methanol (50 ml) and added aluminum chloride (0.003 mol) with 2-3 drops of conc. hydrochloric acid as catalyst amount then the reaction mixture was refluxed for 11-12 hrs after that the reaction mixture was cooled to room temperature and poured into crushed ice water with vigorous stirring, filtered and recrystallized with methanol [10].
Step 2: The synthesis of 5-(substituted aldehyde)-7-methyl-3-oxo-N-phenyl-2-((3,4,5,6-tetrahydroxy-tetrahydro-2H-pyran- 2-yl)methylene)-1,2,3,5-tetrahydroimidazo[1,2-a] pyrimidine-6- carboxamide derivatives: A mixture of intermediate-1 (0.01 mol), sodium benzoate (2 g), dextrose (0.01 mol), glacial acetic acid (20 ml), ethyl acetoacetate (15 ml) and monochloroacetic acid (0.015 mol) were taken in RBF and refluxed with controlled temperature at 140-142°C for 6-7 hrs. The reaction mixture was cooled at room temperature and poured into ice cold water to yielded solid precipitate or titled compounds (1-3), filtered and recrystallized with methanol.
Compound 1 (7-Methyl-3-oxo-N-phenyl-2-((3,4,5,6- tetrahydroxy-tetrahydro-2H-pyran-2-yl)methylene)-5-(3,4,5- trimethoxyphenyl)-1,2,3,5-tetrahydroimidazo[1,2-a]pyrimidine-6- carboxamide): IR (KBr pellets, cm-1): 3062 (C-H str., phenyl nucleus), 1596 (C=C str., phenyl nucleus), 694 (C-C str., phenyl nucleus), 1630 (C=O str.,), 3321 (N-H str., 2˚amide), 1630 (N=CH str., pyrimidine), 1244 (C-N str., pyrimidine), 2779 (C-H str., cyclic ether), 1126 (C-O-C str., aryl ether), 3321 (O-H str., polyhydroxy on dextrose), 1244 (C-O-C str., OCH3); 1H NMR (DMSO-d6, δppm): 7.45-7.49 (m, 7H, Ar-H), 2.10 (s, 1H, NH, amide), 8.25 (s, 1H, NH, amide), 3.86-4.22 (m, 5H, CH, tetrahydropyran), 2.10 (m, 4H, OH alcohol), 3.86 (m, 9H, OCH3).
Synthesis, Biological Evaluation and Validation Studies of Novel 5-(Substituted Aldehyde)-2-imino-7-methyl-3-oxo-N-phenyl-1,2,3,5-tetrahydroimidazo[1,2-a]pyrimidine-6-carboxamide Scaffolds
| Jyoti Rani, Monika Saini, Sanjiv Kumar and Prabhakar Kumar Verma* | |
| Department of Pharmaceutical Sciences, Maharshi Dayanand University, Rohtak-124 001, Haryana, India | |
| *Corresponding Author : | Prabhakar Kumar Verma Department of Pharmaceutical Sciences Maharshi Dayanand University Rohtak-124 001, Harayana, India Tel: 9992581437 E-mail: vermapk422@rediffmail.com |
| Received March 07, 2016; Accepted March 29, 2016; Published March 31, 2016 | |
| Citation: Rani J, Saini M, Kumar S, Verma PK (2016) Synthesis, Biological Evaluation and Validation Studies of Novel 5-(Substituted Aldehyde)-2-imino-7-methyl-3-oxo-N-phenyl-1,2,3,5-tetrahydroimidazo[1,2-a]pyrimidine-6-carboxamide Scaffolds. Med chem (Los Angeles) 6:218-223. doi:10.4172/2161-0444.1000349 | |
| Copyright: © 2016 Rani J, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
link
///////////// 5-(Substituted Aldehyde)-2-imino-7-methyl-3-oxo-N-phenyl-1,2,3,5-tetrahydroimidazo[1,2-a]pyrimidine-6-carboxamide, Scaffolds













Sorry, the comment form is closed at this time.