
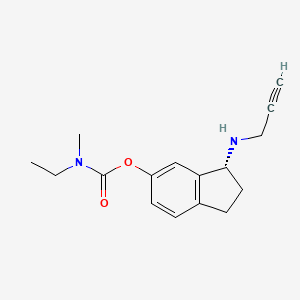
Ladostigil, TV-3,326
(N-propargyl-(3R) aminoindan-5yl)-ethyl methyl carbamate
(3R)-3-(Prop-2-ynylamino)indan-5-yl ethyl(methyl)carbamate; R-CPAI
Carbamic acid, ethylmethyl-, (3R)-2,3-dihydro-3-(2-propynylamino)-1H-inden-5-yl ester
Condition(s): Mild Cognitive Impairment
U.S. FDA Status: Mild Cognitive Impairment (Phase 2)
Company: Avraham Pharmaceuticals Ltd
Target Type: Cholinergic System
| CAS No: |
209349-27-4 |
| Synonyms: |
Ladostigil, TV-3326, UNII-SW3H1USR4Q |
| Molecular Weight: |
272.346 g/mol |
|
|
| Chemical Formula: |
C16-H20-N2-O2 |
|
|
| IUPAC Name: |
(3R)-3-(Prop-2-ynylamino)indan-5-yl ethyl(methyl)carbamate N-Propargyl-(3R)-aminoindan-5-yl) ethyl methyl carbamate |
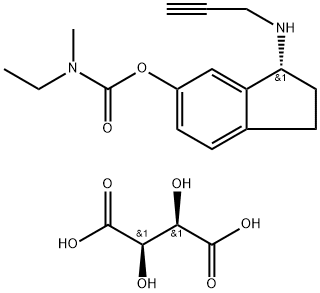
CAS 209394-46-7, Ladostigil tartrate
N-Ethyl-N-methylcarbamic acid 3(R)-(2-propynylamino)-2,3-dihydro-1H-inden-5-yl ester L-tartrate
In 2010, ladostigil tartrate was licensed by Technion Research & Development Foundation and Yissum to Avraham for the treatment of Alzheimer’s disease and other neurogenerative diseases.
Ladostigil (TV-3,326) is a novel neuroprotective agent being investigated for the treatment of neurodegenerative disorders likeAlzheimer’s disease, Lewy body disease, and Parkinson’s disease.[1] It acts as a reversible acetylcholinesterase andbutyrylcholinesterase inhibitor, and an irreversible monoamine oxidase B inhibitor, and combines the mechanisms of action of older drugs like rivastigmine and rasagiline into a single molecule.[2][3] In addition to its neuroprotective properties, ladostigil enhances the expression of neurotrophic factors like GDNF and BDNF, and may be capable of reversing some of the damage seen in neurodegenerative diseases via the induction of neurogenesis.[4] Ladostigil also has antidepressant effects, and may be useful for treating comorbid depression and anxiety often seen in such diseases as well.[5][6]
Ladostigil [(N-propargyl-(3R) aminoindan-5yl)-ethyl methyl carbamate] is a dual acetylcholine-butyrylcholineesterase and brain selective monoamine oxidase (MAO)-A and -B inhibitor in vivo (with little or no MAO inhibitory effect in the liver and small intestine), intended for the treatment of dementia co-morbid with extrapyramidal disorders and depression (presently in a Phase IIb clinical study). This suggests that the drug should not cause a significant potentiation of the cardiovascular response to tyramine, thereby making it a potentially safer antidepressant than other irreversible MAO-A inhibitors. Ladostigil was shown to antagonize scopolamine-induced impairment in spatial memory, indicating that it can cause significant increases in rat brain cholinergic activity. Furthermore, ladostigil prevented gliosis and oxidative-nitrative stress and reduced the deficits in episodic and spatial memory induced by intracerebroventricular injection of streptozotocin in rats. Ladostigil was demonstrated to possess potent anti-apoptotic and neuroprotective activities in vitro and in various neurodegenerative rat models, (e.g. hippocampal damage induced by global ischemia in gerbils and cerebral oedema induced in mice by closed head injury). These neuroprotective activities involve regulation of amyloid precursor protein processing; activation of protein kinase C and mitogen-activated protein kinase signaling pathways; inhibition of neuronal death markers; prevention of the fall in mitochondrial membrane potential and upregulation of neurotrophic factors and antioxidative activity. Recent findings demonstrated that the major metabolite of ladostigil, hydroxy-1-(R)-aminoindan has also a neuroprotective activity and thus, may contribute to the overt activity of its parent compound. This review will discuss the scientific evidence for the therapeutic potential use of ladostigil in Alzheimer’s and Lewy Body diseases and the molecular signaling pathways that are considered to be involved in the biological activities of the drug
PAPER
Tetrahedron: Asymmetry (2012), 23(5), 333-338
http://www.sciencedirect.com/science/article/pii/S0957416612001334

 (R)-3-(Prop-2-ynylamino)-2,3-dihydro-1H-inden-5-yl ethyl(methyl)carbamate (R)-3-(Prop-2-ynylamino)-2,3-dihydro-1H-inden-5-yl ethyl(methyl)carbamate
C16H20N2O2 |
ee: 89%
 (c 1.46, CHCl3) (c 1.46, CHCl3)
Source of chirality: the precursor
Absolute configuration: (R)
|
Yona Geffen CEO
Avraham Pharmaceuticals Ltd.
42 Hayarkon st.
Northern Industrial Zone
Yavneh, 81227
Israel
| WO1998027055A1 * |
18 Dec 1997 |
25 Jun 1998 |
Teva Pharmaceutical Industries, Ltd. |
Aminoindan derivatives |
| WO2005051371A1 |
28 Sep 2004 |
9 Jun 2005 |
Technion Research & Development Foundation Ltd. |
Compositions and methods for treatment of cardiovascular disorders and diseases |
| WO2006130726A2 |
31 May 2006 |
7 Dec 2006 |
Teva Pharmaceutical Industries, Ltd. |
Use of ladostigil for the treatment of multiple sclerosis |
| WO2007087029A2 * |
11 Dec 2006 |
2 Aug 2007 |
Yissum Research Development Company Of The Hebrew University Of Jerusalem |
Use of low-dose ladostigil for neuroprotection |
| WO2009022345A1 |
14 Aug 2008 |
19 Feb 2009 |
Yissum Research Development Company Of The Hebrew University Of Jerusalem |
Phenyl carbamates for the treatment of multiple sclerosis |
| WO2009022346A2 |
14 Aug 2008 |
19 Feb 2009 |
Yissum Research Development Company Of The Hebrew University Of Jerusalem |
Phenyl carbamates for treating gastrointestinal inflammation |
| WO2012059920A1 |
2 Nov 2011 |
10 May 2012 |
Yissum Research Development Company Of The Hebrew University Of Jerusalem Ltd. |
Ladostigil dosage regime |
| US6251938 |
18 Jun 1999 |
26 Jun 2001 |
Teva Pharmaceutical Industries, Ltd., |
Phenylethylamine derivatives |
| US6303650 |
18 Jun 1999 |
16 Oct 2001 |
Yissum Research Development Company Of The Hebrew University Of Jerusalem |
Aminoindan derivatives |
| US6538025 |
31 Aug 2001 |
25 Mar 2003 |
Teva Pharmaceutical Industries, Ltd. |
Aminoindan derivatives |
| US7335685 |
22 Feb 2006 |
26 Feb 2008 |
Teva Pharmaceutical Industries, Ltd. |
Crystals of ladostigil tartrate, methods of production and pharmaceutical compositions thereof |
| US7375249 |
21 Feb 2006 |
20 May 2008 |
Teva Pharmaceutical Industries Ltd. |
Process for the synthesis of enantiomeric indanylamine derivatives |
| US7476757 |
15 Apr 2008 |
13 Jan 2009 |
Teva Pharmaceutical Industries Ltd. |
Process for the synthesis of enantiomeric indanylamine derivatives |
| US7491847 |
15 Nov 2006 |
17 Feb 2009 |
Teva Pharmaceutical Industries, Ltd. |
Methods for isolating propargylated aminoindans |
| US20050222123 |
27 Jan 2005 |
6 Oct 2005 |
North Shore-Long Island Jewish Research Institute |
Cholinesterase inhibitors for treating inflammation |
| US20060189685 |
24 Feb 2006 |
24 Aug 2006 |
Daniella Licht |
Formulations of ladostigil tartrate |
| US20060189819 |
22 Feb 2006 |
24 Aug 2006 |
Teva Pharmaceutical Industries, Ltd. |
Crystals of ladostigil tartrate, methods of production and pharmaceutical compositions thereof |
| US20060199974 |
21 Feb 2006 |
7 Sep 2006 |
Teva Pharmaceutical Industries Ltd. |
Process for the synthesis of enantiomeric indanylamine derivatives |
| US20070088082 |
28 Sep 2006 |
19 Apr 2007 |
Judith Aronhime |
Polymorphic forms of ladostigil tartrate |
| US20070093549 |
28 Sep 2006 |
26 Apr 2007 |
Judith Aronhime |
Methods for preparation of ladostigil tartrate crystalline form A1 |
| US20070112217 |
15 Nov 2006 |
17 May 2007 |
Anton Frenkel |
Methods for isolating propargylated aminoindans |
| US20070135518 |
8 Dec 2006 |
14 Jun 2007 |
Marta Weinstock-Rosin |
Use of low-dose ladostigil for neuroprotection |
| US20070203232 |
23 Feb 2007 |
30 Aug 2007 |
Victor Piryatinsky |
Propargylated aminoindans, processes for preparation, and uses thereof |
| US20070232691 |
28 Mar 2007 |
4 Oct 2007 |
Tamar Goren |
Use of ladostigil for the treatment of schizophrenia |
| US20070293583 |
11 Dec 2006 |
20 Dec 2007 |
Marta Weinstock-Rosin |
Use of low-dose ladostigil for neuroprotection |
| US5532415 * |
Mar 28, 1995 |
Jul 2, 1996 |
Teva Pharmaceutical Industries Ltd. |
R-enantiomer of N-propargyl-1-aminoindan, salts, compositions and uses thereof |
| US5703059 * |
Jan 19, 1994 |
Dec 30, 1997 |
British Biotech Pharmaceuticals Ltd. |
Disaccharide ligands for selectins |
| US5936000 * |
Jan 16, 1996 |
Aug 10, 1999 |
Pharmacia & Upjohn Company |
2-aminoindans as selective dopamine D3 ligands |
| US6271261 * |
Jun 24, 1997 |
Aug 7, 2001 |
Smithkline Beecham Corporation |
IL-8 receptor antagonists |
| US6271263 * |
Mar 2, 1999 |
Aug 7, 2001 |
Teva Pharmaceutical Industries, Ltd. |
Compositions containing and methods of using 1-aminoindan and derivatives thereof and process for preparing optically active 1-aminoindan derivatives |
| US6303650 * |
Jun 18, 1999 |
Oct 16, 2001 |
Yissum Research Development Company Of The Hebrew University Of Jerusalem |
Aminoindan derivatives |
| US6462222 * |
Aug 31, 2001 |
Oct 8, 2002 |
Yissum Research Development Company Of The Hebrew University Of Jerusalem |
Aminoindan derivatives |
| US6538025 * |
Aug 31, 2001 |
Mar 25, 2003 |
Teva Pharmaceutical Industries, Ltd. |
Aminoindan derivatives |
| US6737547 * |
Sep 15, 1999 |
May 18, 2004 |
Teva Pharmaceutical Industries, Ltd. |
Compositions containing and methods of using N-acyl-1H-aminoindenes |
| US20040010038 * |
Feb 27, 2003 |
Jan 15, 2004 |
Eran Blaugrund |
Propargylamino indan derivatives and propargylamino tetralin derivatives as brain-selective MAO inhibitors |
| Citing Patent |
Filing date |
Publication date |
Applicant |
Title |
| US7649115 |
Jun 1, 2006 |
Jan 19, 2010 |
Jenrin Discovery, Inc. |
MAO-B inhibitors useful for treating obesity |
| US8541475 |
Dec 31, 2009 |
Sep 24, 2013 |
Jenrin Discovery, Inc. |
MAO-B inhibitors useful for treating obesity |
| US8569545 |
Jun 2, 2009 |
Oct 29, 2013 |
Generics (Uk) Limited |
Process for the preparation of enantiomerically pure amines |
| US8809589 |
Jul 18, 2013 |
Aug 19, 2014 |
Generics [Uk] Limited |
Process for the preparation of enantiomerically pure amines |
| US20070088004 * |
Jun 1, 2006 |
Apr 19, 2007 |
Mcelroy John F |
MAO-B inhibitors useful for treating obesity |
| US20100168068 * |
Dec 31, 2009 |
Jul 1, 2010 |
Jenrin Discovery |
Mao-b inhibitors useful for treating obesity |
| US20110184071 * |
Jun 2, 2009 |
Jul 28, 2011 |
Vinayak Gore |
process for the preparation of amines |
| US20110218360 * |
|
Sep 8, 2011 |
Dr. Reddy’s Laboratories Ltd. |
Preparation of rasagiline and salts thereof |
| CN103443111A * |
Apr 2, 2012 |
Dec 11, 2013 |
高砂香料工业株式会社 |
Novel ruthenium complex and process for producing optically active alcohol compound using same as catalyst |
| CN103443111B * |
Apr 2, 2012 |
Mar 2, 2016 |
高砂香料工业株式会社 |
钌配合物以及以该配合物作为催化剂的光学活性醇化合物的制备方法 |
| WO2013118126A1 |
Feb 11, 2013 |
Aug 15, 2013 |
Yissum Research Development Company Of The Hebrew University Of Jerusalem Ltd. |
Ladostigil therapy for immunomodulation |
///////////Ladostigil, TV-3,326
c1c(cc2c(c1)CC[C@H]2NCC#C)OC(=O)N(CC)C



















 (R)-3-(Prop-2-ynylamino)-2,3-dihydro-1H-inden-5-yl ethyl(methyl)carbamate
(R)-3-(Prop-2-ynylamino)-2,3-dihydro-1H-inden-5-yl ethyl(methyl)carbamate
 (R)-3-(Prop-2-ynylamino)-2,3-dihydro-1H-inden-5-yl ethyl(methyl)carbamate hemi((2R,3R)-2,3-dihydroxysuccinate)
(R)-3-(Prop-2-ynylamino)-2,3-dihydro-1H-inden-5-yl ethyl(methyl)carbamate hemi((2R,3R)-2,3-dihydroxysuccinate)


Sorry, the comment form is closed at this time.