Jun 012016
Catal. Sci. Technol., 2016, 6,3378-3385
DOI: 10.1039/C6CY00033A, Paper
DOI: 10.1039/C6CY00033A, Paper
Gang Yang, Lijun Zhou
Several key issues regarding the mechanisms of proline catalysis are unravelled by first-principles calculations that can guide future catalyst design.
Several key issues regarding the mechanisms of proline catalysis are unravelled by first-principles calculations that can guide future catalyst design.
Mechanisms and reactivity differences of proline-mediated catalysis in water and organic solvents
*Corresponding authors
aCollege of Resource and Environment & Chongqing Key Laboratory of Soil Multi-scale Interfacial Process, Southwest University, Chongqing, PR China
E-mail: theobiochem@gmail.com
Fax: +86 023 68250444
Tel: +86 023 68251545
E-mail: theobiochem@gmail.com
Fax: +86 023 68250444
Tel: +86 023 68251545
Catal. Sci. Technol., 2016,6, 3378-3385
DOI: 10.1039/C6CY00033A
http://pubs.rsc.org/en/Content/ArticleLanding/2016/CY/C6CY00033A?utm_source=feedburner&utm_medium=feed&utm_campaign=Feed%3A+rss%2Fcy+%28RSC+-+Catalysis+Science+%26+Technology+latest+articles%29#!divAbstract
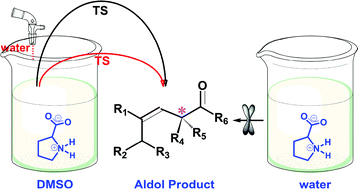
Proline is an efficient and versatile catalyst for organic reactions while a number of issues remain controversial. Here, ab initio and density functional calculations were used to unravel a few key issues of catalytic mechanisms in water and organic solvents. Zwitterionic proline that predominates in water and DMSO is assumed to be the active conformation for catalysis, and reactivity differences in two solvents are revealed. Meanwhile, an abundance of experimental observations can be finely interpreted by the present computational results, including those seemingly contradictory. Although bearing lower activation barriers than that in DMSO, the production of enamines and further aldol products in water will be blocked at an early stage (J. Am. Chem. Soc., 2006, 128, 734) because the reaction in water is significantly driven towards acetyl formation that is kinetically and thermodynamically preferred. Due to significant promotion of the rate-determining proton transfer step, aldol reactions in organic solvents can be obviously initiated by the addition of some water (Angew. Chem., Int. Ed., 2004, 43, 1983). In order to show catalytic effects in water (an obviously environmentally benign solvent), proline has to be structurally modified so that canonical structures can be the principal (or sole) conformations, which is in line with the analyses of all proline-based catalysts available in water (e.g., J. Am. Chem. Soc., 2006, 128, 734, Catal. Commun., 2012, 26, 6). Thus, the present results provide insightful clues to mechanisms of proline-mediated catalysis as well as future design of more efficient catalysts.
IF YOU HAVE ENJOYED IT ………EMAIL ME amcrasto@gmail.com, +919323115463, India

INDIA FLAG

DR ANTHONY CRASTO , WORLDDRUGTRACKER, HELPING MILLIONS, MAKING INDIA AND INDIANS PROUD
//////Mechanisms, reactivity, differences, proline-mediated catalysis, water , organic solvents













Sorry, the comment form is closed at this time.