

Higenamine Hydrochloride
- 6,7-Isoquinolinediol, 1,2,3,4-tetrahydro-1-[(4-hydroxyphenyl)methyl]-, hydrochloride (9CI)
- 6,7-Isoquinolinediol, 1,2,3,4-tetrahydro-1-[(4-hydroxyphenyl)methyl]-, hydrochloride, (±)-
- (±)-Demethylcoclaurine hydrochloride
NDA Filed in china
A β-adrenoceptor partial agonist potentially for the treatment of coronary heart disease.


CAS No.11041-94-4 (Higenamine hydrochloride)

CAS 5843-65-2(free)
Higenamine (norcoclaurine) is a chemical compound found in a variety of plants including Nandina domestica (fruit), Aconitum carmichaelii (root), Asarum heterotropioides, Galium divaricatum (stem and vine), Annona squamosa, and Nelumbo nucifera (lotus seeds).
Legality
Higenamine, also known as norcoclaurine HCl, is legal to use within food supplements in the UK, EU, the USA and Canada. but banned use in The NCAA. Its main is within food supplements developed for weight management, also known as ‘fat burners’. However, products containing (or claiming to contain) pharmacological relevant quantities still require registration as a medicine. The regulatory boundaries for higenamine are unclear as modern formulations have not been clinically evaluated. Traditional formulations with higenamine have been used for thousands of years within Chinese medicine and come from a variety of sources including fruit and orchids. There are no studies comparing the safety of modern formulations (based on synthetic higenamine) with traditional formulations. Nevertheless, it will not be added to the EU ‘novel foods’ catalogue, which details all food supplements that require a safety assessment certificate before use.[1]

Pharmacology
Since higenamine is present in plants which have a history of use in traditional medicine, the pharmacology of this compound has attracted scientific interest. A variety of effects have been observed in in vitro studies and in animal models, but its effects in humans are unknown.
The results of a 2009 study exposed the compound as a β2 adrenergic receptor agonist.[2]
In animal models, higenamine has been demonstrated to be a β2 adrenoreceptor agonist.[2][3][4][5][6] Adrenergic receptors, or adrenoceptors, belong to the class of G protein–coupled receptors, and are the most prominent receptors in the adipose membrane, besides also being expressed in skeletal muscle tissue. These adipose membrane receptors are classified as either α or β adrenoceptors. Although these adrenoceptors share the same messenger, cyclic adenosine monophosphate (cAMP), the specific transduction pathway depends on the receptor type (α or β). Higenamine partly exerts its actions by the activation of an enzyme,adenylate cyclase, responsible for boosting the cellular concentrations of the adrenergic second messenger, cAMP.[7]
In a rodent model, it was found that higenamine produced cardiotonic, vascular relaxation, and bronchodilator effects.[8][9] In particular, higenamine, via a beta-adrenoceptor mechanism, induced relaxation in rat corpus cavernosum, leading to improved vasodilation and erectile function.
Related to improved vasodilatory signals, higenamine has been shown in animal models to possess antiplatelet and antithrombotic activity via a cAMP-dependent pathway, suggesting higenamine may contribute to enhanced vasodilation and arterial integrity.[2][7][9][10]
Toxicity
Regarding toxicity, researchers have suggested that the levels of higenamine reported in food consumption (estimated 47.5 mg in a 9-ounce serving of Lotus) would be comparable to the amount used in food supplements.[citation needed] Higenamine is a beta-adrenergic agonist which has effects on the function of trachea and heart muscles.[11][12]During a study of acute toxicity, mice were orally administered the compound at a dose of 2 g per kg of bodyweight. No mice died during the study.[13] higenamine is one of the main chemicals in a plant called aconite. Aconite has been shown to cause serious heart-related side effects including arrhythmias and even death. in some sources of HIGENAMINE from certain plants that have Aconite
PAPER
Chemical & Pharmaceutical Bulletin (1978), 26(7), 2284-5
https://www.jstage.jst.go.jp/article/cpb1958/26/7/26_7_2284/_pdf
PATENT
CN 103554022
http://google.com/patents/CN103554022B?cl=en
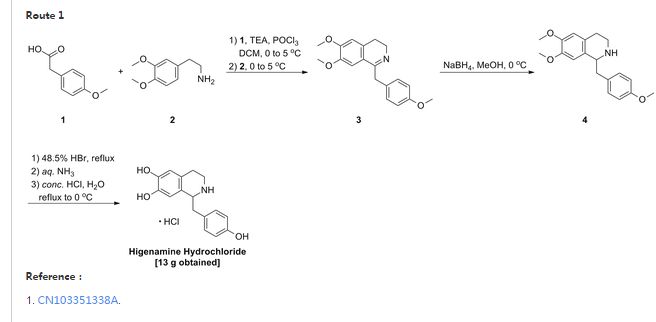
Example 1:
[0024] to the S-necked flask 200mL of anhydrous ammonia clever four furans, lOg instrument crumbs, olive mix was added 0. 5g ship, continue to embrace the mix was added 10 minutes after which 2 drops of 1,2-B burning desert, Continue mixing until the reaction mixture embrace color disappeared, the reaction was cooled to square ° C, and slowly mixed solution thereto 31. 6g4- methoxy Desert Festival and 50mL tetraammine clever furans dropped, about 60min addition was complete, the reaction fluid continues to cool to -65 ° C, to which was slowly dropping 20 percent, 7-dimethoxy-3,4-diamine different wow beep and a mixed solution of ammonia lOOmL four clever furans, the addition was complete continue to maintain – 65 ° C for 2 hours after the embrace slowly warmed 0 ° C, maintaining the internal temperature of 100 ° C 〇 blood slowly added to the reaction mixture, the addition was completed adding 200 blood continues to embrace mixed with ethyl acetate after 0.5 hours, allowed to stand liquid separation, organic phase was separated, dried over anhydrous sulfate steel, concentrated to afford 6, 7-dimethoxy -l- (4- methoxy section yl) -1,2, 3, 4-isopropyl tetraammine wow toot 24. 9g, a yield of 76.1%.
[00 Qiao] to the reaction flask prepared above 6, 7-dimethoxy -l- (4- methoxybenzyl) -1,2, 3, 4 tetraammine different wow beep 24. 9g , 47% aqueous ammonia desert 200 blood acid heated to 130 ° C reflux of cooled to room temperature, precipitation of large amount of solid, filtered higenamine ammonia salt desert, the solid was added 1. of water and continue to add 50 Blood mixed with ammonia football ground, filtered higenamine to higenamine was added lL4mol / L aqueous hydrochloric acid, 80 ° C heat to embrace mixed, cooled to 25 ° C filtration and drying to obtain a final product hydrochloric acid higenamine 11. 7g, a yield of 73.3%.
References
- http://ec.europa.eu/food/food/biotechnology/novelfood/novel_food_catalogue_en.htm
- Tsukiyama, M; Ueki, T; Yasuda, Y; Kikuchi, H; Akaishi, T; Okumura, H; Abe, K (2009). “Beta2-adrenoceptor-mediated tracheal relaxation induced by higenamine from Nandina domestica Thunberg”. Planta Medica 75 (13): 1393–9. doi:10.1055/s-0029-1185743. PMID 19468973.
- Kashiwada, Y; Aoshima, A; Ikeshiro, Y; Chen, YP; Furukawa, H; Itoigawa, M; Fujioka, T; Mihashi, K; et al. (2005). “Anti-HIV benzylisoquinoline alkaloids and flavonoids from the leaves of Nelumbo nucifera, and structure-activity correlations with related alkaloids”.Bioorganic & Medicinal Chemistry 13 (2): 443–8. doi:10.1016/j.bmc.2004.10.020.PMID 15598565.
- Kimura, I; Chui, LH; Fujitani, K; Kikuchi, T; Kimura, M (1989). “Inotropic effects of (+/-)-higenamine and its chemically related components, (+)-R-coclaurine and (+)-S-reticuline, contained in the traditional sino-Japanese medicines “bushi” and “shin-i” in isolated guinea pig papillary muscle”. Japanese journal of pharmacology 50 (1): 75–8.doi:10.1254/jjp.50.75. PMID 2724702.
- Kang, YJ; Lee, YS; Lee, GW; Lee, DH; Ryu, JC; Yun-Choi, HS; Chang, KC (1999). “Inhibition of activation of nuclear factor kappaB is responsible for inhibition of inducible nitric oxide synthase expression by higenamine, an active component of aconite root”. The Journal of Pharmacology and Experimental Therapeutics 291 (1): 314–20.PMID 10490919.
- Yun-Choi, HS; Pyo, MK; Park, KM; Chang, KC; Lee, DH (2001). “Anti-thrombotic effects of higenamine”. Planta Medica 67 (7): 619–22. doi:10.1055/s-2001-17361.PMID 11582538.
- Kam, SC; Do, JM; Choi, JH; Jeon, BT; Roh, GS; Chang, KC; Hyun, JS (2012). “The relaxation effect and mechanism of action of higenamine in the rat corpus cavernosum”.International Journal of Impotence Research 24 (2): 77–83. doi:10.1038/ijir.2011.48.PMID 21956762.
- Bai, G; Yang, Y; Shi, Q; Liu, Z; Zhang, Q; Zhu, YY (2008). “Identification of higenamine in Radix Aconiti Lateralis Preparata as a beta2-adrenergic receptor agonist1”. Acta pharmacologica Sinica 29 (10): 1187–94. doi:10.1111/j.1745-7254.2008.00859.x.PMID 18817623.
- Pyo, MK; Lee, DH; Kim, DH; Lee, JH; Moon, JC; Chang, KC; Yun-Choi, HS (2008). “Enantioselective synthesis of (R)-(+)- and (S)-(-)-higenamine and their analogues with effects on platelet aggregation and experimental animal model of disseminated intravascular coagulation”. Bioorganic & Medicinal Chemistry Letters 18 (14): 4110–4.doi:10.1016/j.bmcl.2008.05.094. PMID 18556200.
- Liu, W; Sato, Y; Hosoda, Y; Hirasawa, K; Hanai, H (2000). “Effects of higenamine on regulation of ion transport in guinea pig distal colon”. Japanese journal of pharmacology 84(3): 244–51. doi:10.1254/jjp.84.244. PMID 11138724.
- Wong, KK; Lo, CF; Chen, CM (1997). “Endothelium-dependent higenamine-induced aortic relaxation in isolated rat aorta”. Planta Medica 63 (2): 130–2. doi:10.1055/s-2006-957628. PMID 9140225.
- Ueki, T; Akaishi, T; Okumura, H; Morioka, T; Abe, K (2011). “Biphasic tracheal relaxation induced by higenamine and nantenine from Nandina domestica Thunberg”. Journal of pharmacological sciences 115 (2): 254–7. doi:10.1254/jphs.10251sc. PMID 21282929.
- Lo, CF; Chen, CM (1997). “Acute toxicity of higenamine in mice”. Planta Medica 63 (1): 95–6. doi:10.1055/s-2006-957619. PMID 9063102.
banned in ncaa https://www.ncaa.org/sites/default/files/2015-16%20NCAA%20Banned%20Drugs.pdf
| CN1539823A * | Oct 27, 2003 | Oct 27, 2004 | 中国医学科学院药物研究所 | Method for preparing new demethyl conclaurine and medinal salt |
| CN1764647A * | Mar 23, 2004 | Apr 26, 2006 | 埃科特莱茵药品有限公司 | Tetrahydroisoquinolyl acetamide derivatives for use as orexin receptor antagonists |
| CN103351338A * | Jun 17, 2013 | Oct 16, 2013 | 张家港威胜生物医药有限公司 | Simple preparation process of higenamine hydrochloride |
| US20060030586 * | Sep 27, 2004 | Feb 9, 2006 | Education Center Of Traditional Chinese Medicine Co. | Method and health food for preventing and/or alleviating psychiatric disorder, and/or for effectuating sedation |
| WO2011038169A2 * | Sep 24, 2010 | Mar 31, 2011 | Mallinckrodt Inc. | One-pot preparation of hexahydroisoquinolines from amides |
 |
|
| Names | |
|---|---|
| IUPAC name
1-[(4-Hydroxyphenyl)methyl]-1,2,3,4-tetrahydroisoquinoline-6,7-diol
|
|
| Other names
norcoclaurine, demethylcoclaurine
|
|
| Identifiers | |
| 5843-65-2 106032-53-5 (R) 22672-77-1 (S) |
|
| ChEBI | CHEBI:18418 |
| ChEMBL | ChEMBL19344 |
| ChemSpider | 102800 |
| Jmol 3D model | Interactive image |
| KEGG | C06346 |
| MeSH | higenamine |
| PubChem | 114840 |
| Properties | |
| C16H17NO3 | |
| Molar mass | 271.32 g·mol−1 |
/////













Sorry, the comment form is closed at this time.