May 212016
Green Chem., 2016, Advance Article
DOI: 10.1039/C6GC00924G, Paper
DOI: 10.1039/C6GC00924G, Paper
Arvind K. Yadav, Lal Dhar S. Yadav
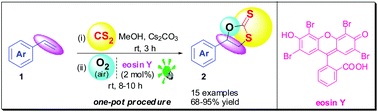
An efficient, one-pot, highly regioselective synthesis of 1,3-oxathiolane-2-thiones from styrenes, CS2, atmospheric O2 and visible light is reported.
An efficient, one-pot, highly regioselective synthesis of 1,3-oxathiolane-2-thiones from styrenes, CS2, atmospheric O2 and visible light is reported.
Eosin Y catalyzed difunctionalization of styrenes using O2 and CS2: a direct access to 1,3-oxathiolane-2-thiones
Paper
Eosin Y catalyzed difunctionalization of styrenes using O2 and CS2: a direct access to 1,3-oxathiolane-2-thiones
*Corresponding authors
aGreen Synthesis Lab, Department of Chemistry, University of Allahabad, Allahabad-211002, India
E-mail: ldsyadav@hotmail.com
Fax: +91 5322460533
Tel: +91 5322500652
E-mail: ldsyadav@hotmail.com
Fax: +91 5322460533
Tel: +91 5322500652
Green Chem., 2016, Advance Article
DOI: 10.1039/C6GC00924G
Visible light promoted straightforward highly regioselective synthesis of 1,3-oxathiolane-2-thiones (cyclic dithiocarbonates) starting directly from styrenes, CS2 and air (O2) is reported. The protocol utilizes eosin Y as an organophotoredox catalyst and clean resources like visible light and air (O2) as sustainable reagents at room temperature in a one-pot procedure. Additionally, the approach is advantageous in terms of step economy as it skips the prefunctionalization of styrenes to oxiranes, which has been inevitable in commonly used syntheses of 1,3-oxathiolane-2-thiones.
//////////Eosin Y, catalyzed, difunctionalization, styrenes, O2, CS2, 1,3-oxathiolane-2-thiones














Sorry, the comment form is closed at this time.