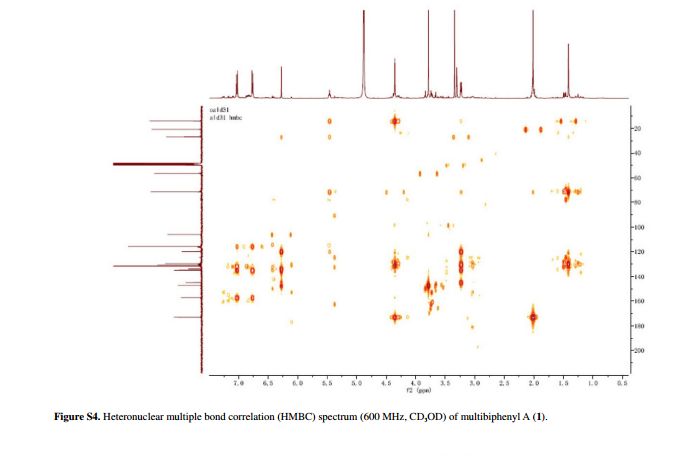
Figure 2 Selected HMBC (H→C) and 1H-1H correlation spectroscopy (COSY) (–) correlations of 1.
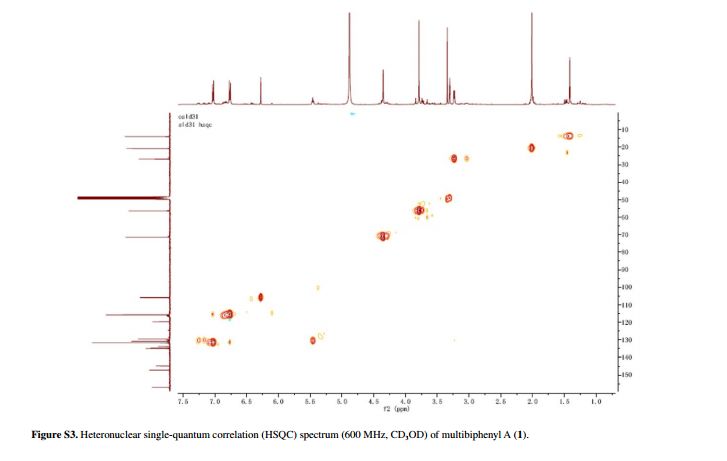
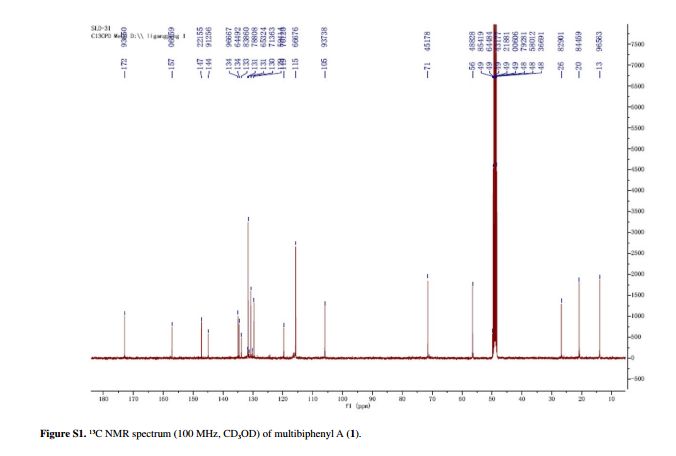
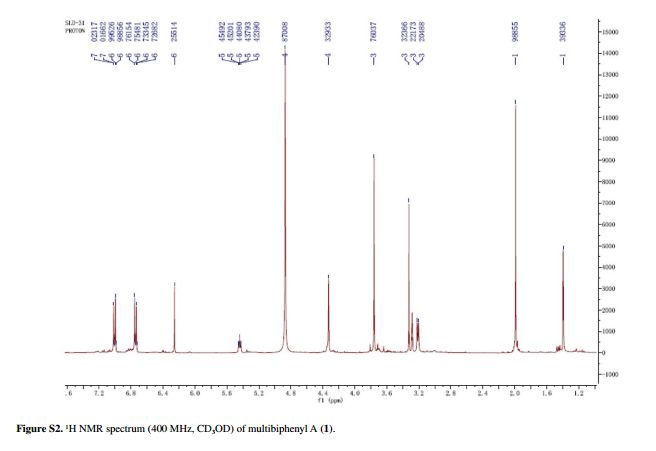
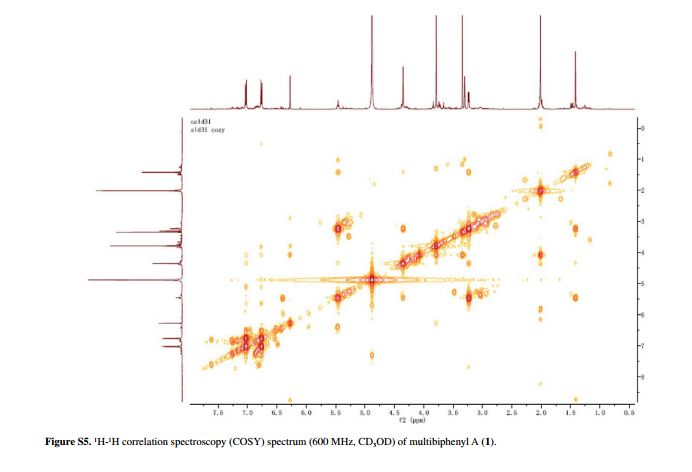
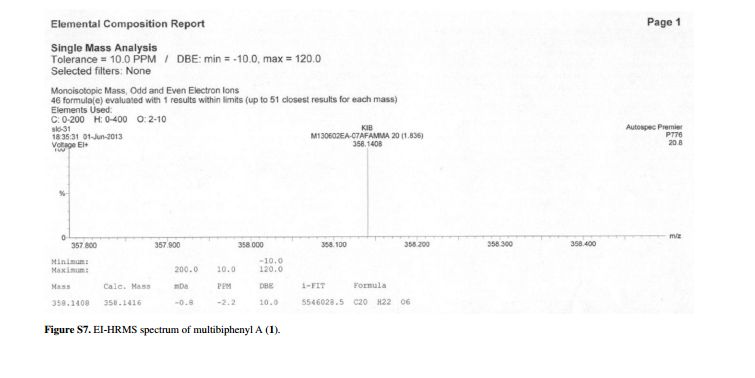
Compound 1 was obtained as a pale yellow gum. The molecular formula was determined to be C20H22O6 from the molecular ion peak [M]+ at m/z 358.1408 in the EI-HRMS. The IR spectrum indicated that 1 possesses hydroxy (3422 cm-1), phenyl (2939, 1498 cm-1), and carbonyl (1721 cm-1) functional groups. The 1H and 13C NMR spectra (Table 1) revealed the signals for a 1,2,3,4,5-pentasubstituted benzene ring [dH 6.26 (1H, s, H-6); δC 129.6 (C-1), 119.7 (C-2), 144.9 (C-3), 135.0 (C-4), 147.2 (C-5), 105.9 (C-6)], one p-substituted benzene ring [dH 7.00 (2H, dd, J 8.8, 2.4 Hz, H-8, H-12), 6.74 (2H, dd, J 8.8, 2.4 Hz, H-9, H-11); δC 133.8 (C-7), 131.7 (C-8, C-12), 115.7 (C-9, C-11), 157.1 (C-10)], one acetoxyprenyl group [dH 3.21 (2H, d, J 6.7 Hz, H-1′), 5.44 (1H, d, J6.7 Hz, H-2′), 4.33 (2H, s, H-4′), 1.39 (3H, s, H-5′), and 1.99 (3H, s, H-OAc); δC 26.8 (C-1′), 130.7 (C-2′), 134.6 (C-3′), 71.5 (C-4′), 14.0 (C-5′), 172.9, 20.8 (OAc)], and one methoxy group [dH 3.76 (3H, s, OMe-5); δC 56.5 (OMe)], which implied that compound1 was a biphenyl derivative. This conclusion was confirmed by the heteronuclear multiple bond correlation (HMBC) correlations of H-6 with C-7, and of H-8 and H-12 with C-1 (Figure 2). HMBC correlations of H-1′ with C-1, C-2, and C-3, and of H-2′ with C-1 suggested the acetoxyprenyl group at C-2. The methoxy group was located at C-5 from the HMBC correlations of δH 3.76 (OMe) with C-5. Considering the signal for quarternary C-3, C-4, C-10 and the molecular formula of 1, three hydroxy groups were located at C-3, C-4, C-10, respectively. Thus, the structure of 1 was determined as shown (Figure 1), and named multibiphenyl A.
Figure 1 New biphenyls from Garcinia multiflora.
Multibiphenyl A (1)
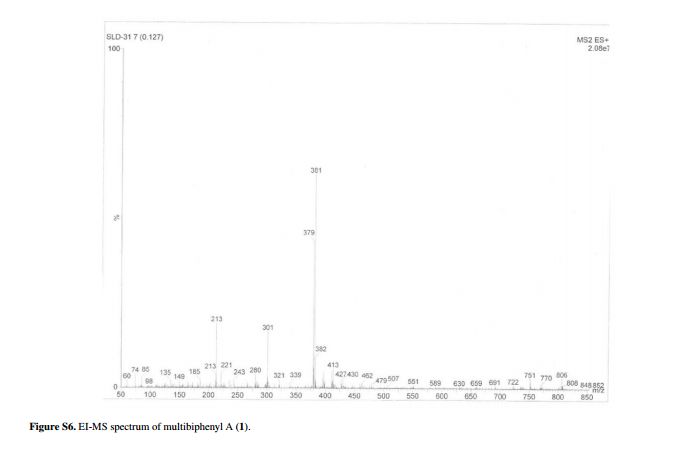
Pale yellow gum; [α]D –11.0 (c 0.07, MeOH); UV (MeOH) lmax / nm (log ε) 570 (2.16), 205 (4.71); IR (KBr) n / cm-1 3422, 2939, 1721, 1611, 1589, 1498, 1443, 1357, 1266, 1172, 1102, 1045, 1023, 838; 1H and 13C NMR data (400 and 100 MHz, CD3OD), see Table 1; ESI-MS (positive mode) m/z 381 [M + Na]+; EI-HRMS (M+) calcd.: 358.1416; found: 358.1408 (C20H22O6).
–11.0 (c 0.07, MeOH); UV (MeOH) lmax / nm (log ε) 570 (2.16), 205 (4.71); IR (KBr) n / cm-1 3422, 2939, 1721, 1611, 1589, 1498, 1443, 1357, 1266, 1172, 1102, 1045, 1023, 838; 1H and 13C NMR data (400 and 100 MHz, CD3OD), see Table 1; ESI-MS (positive mode) m/z 381 [M + Na]+; EI-HRMS (M+) calcd.: 358.1416; found: 358.1408 (C20H22O6).
Table 1 1H and 13C NMR data for compounds 1-3 (d in ppm, 1 and 2 in CD3OD, 3 in CDC13, 100 and 400 MHz)
| No. | 1 | 2 | 3 | |||
|---|---|---|---|---|---|---|
| δC (m) / ppm | δH (m, J , Hz) / ppm | δC (m) / ppm | δH (m, J , Hz) / ppm | δC (m) / ppm | δH (m, J , Hz) / ppm | |
| 1 | 129.6 s | 132.4 s | 132.3 s | |||
| 2 | 119.7 s | 114.2 s | 112.4 s | |||
| 3 | 144.9 s | 142.0 s | 141.5 s | |||
| 4 | 135.0 s | 131.9 s | 132.7 s | |||
| 5 | 147.2 s | 149.6 s | 144.8 s | |||
| 6 | 105.9 d | 6.26 (s, 1H ) | 106.6 d | 6.44 (s, 1H) | 105.5 d | 6.43 (s, 1H) |
| 7 | 133.8 s | 134.6 s | 132.7 s | |||
| 8 | 131.7 d | 7.00 (dd, 1H, J 8.8 Hz, 2.4) | 131.8 d | 7.11 (dd, 1H, J 8.4 Hz, 1.9) | 130.3 d | 7.16 (d, 1H, J 8.6 Hz) |
| 9 | 115.7 d | 6.74 (dd, 1H, J 8.8 Hz, 2.4) | 116.0 d | 6.82 (dd, 1H, J 8.4 Hz, 1.9) | 114.8 d | 6.86 (d, 1H, J 8.6 Hz) |
| 10 | 157.1 s | 157.7 s | 155.5 s | |||
| 11 | 115.7 d | 6.74 (dd, 1H, J 8.8 Hz, 2.4) | 116.0 d | 6.82 (dd, 1H, J 8.4 Hz, 1.9) | 114.8 d | 6.86 (d, 1H, J 8.6 Hz) |
| 12 | 131.7 d | 7.00 (dd, 1H, J 8.8 Hz, 2.4) | 131.8 d | 7.11 (dd, 1H, J 8.4 Hz, 1.9) | 130.3 d | 7.16 (d, 1H, J 8.6 Hz) |
| 1′ | 26.8 t | 3.21 (d, 2H, J 6.7 Hz, CH2) | 124.7 d | 6.41 (d, 1H, J 10.1 Hz) | 21.1 t | 2.59 (t, 2H, J 6.6 Hz, CH2) |
| 2′ | 130.7 d | 5.44 (t, 1H, J 6.7 Hz) | 124.4 d | 5.48 (d, 1H, J 10.1 Hz) | 33.0 t | 1.72 (t, 2H, J 6.6 Hz, CH2) |
| 3′ | 134.6 s | 77.6 s | 74.7 s | |||
| 4′ | 71.5 t | 4.33 (s, 3H, CH3) | 69.0 t | 4.27 (d, 1H, J 11.5 Hz, CH2) | 26.7 q | 1.39 (s, 3H, CH3) |
| 4.14 (d, 1H, J 11.5 Hz, CH2) | ||||||
| 5′ | 14.0 q | 1.39 (s, 3H, CH3) | 23.4 q | 1.47 (s, 3H, CH3) | 26.7 q | 1.39 (s, 3H, CH3) |
| 3-OMe | 56.5 q | 3.76 (s, 3H, OCH3) | 56.5 q | 3.85 (s, 3H, OCH3) | 56.1 q | 3.86 (s, 3H, OCH3) |
| 5′- OAc | 172.9 s | 172.6 s | ||||
| 20.8 q | 1.99 (s, 3H, COCH3) | 20.7 q | 2.00 (s, 3H, COCH3) | |||
Journal of the Brazilian Chemical Society
On-line version ISSN 1678-4790
J. Braz. Chem. Soc. vol.27 no.1 São Paulo Jan. 2016
http://dx.doi.org/10.5935/0103-5053.20150235
ARTICLES
New Biphenyls from Garcinia multiflora
aKey Laboratory of Chemistry in Ethnic Medicinal Resources, State Ethnic Affairs Commission & Ministry of Education, Kunming, P. R. China
bJoint Research Centre for International Cross-Border Ethnic Regions Biomass Clean Utilization in Yunnan, Yunnan Minzu University, 650031 Kunming, P. R. China
cCollege of Resource and Environment, Yuxi Normal University, 653100 Yuxi, P. R. China
Three new biphenyls were isolated from Garcinia multiflora. The structures of these biphenyls were elucidated by spectroscopic methods, and their rotavirus activity was evaluated.
Key words: Garcinia multiflora, biphenyls, anti-rotavirus activity
///////















Sorry, the comment form is closed at this time.