
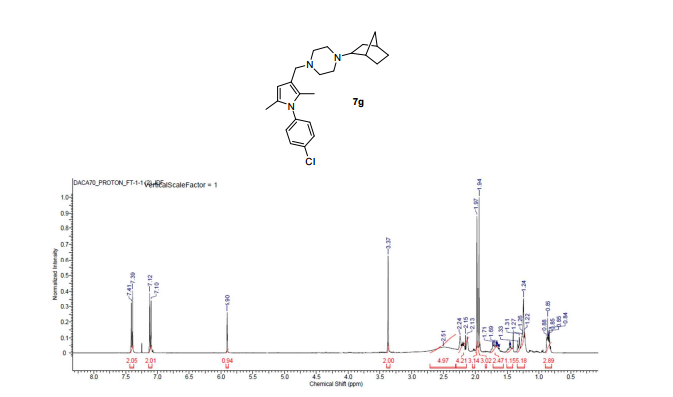
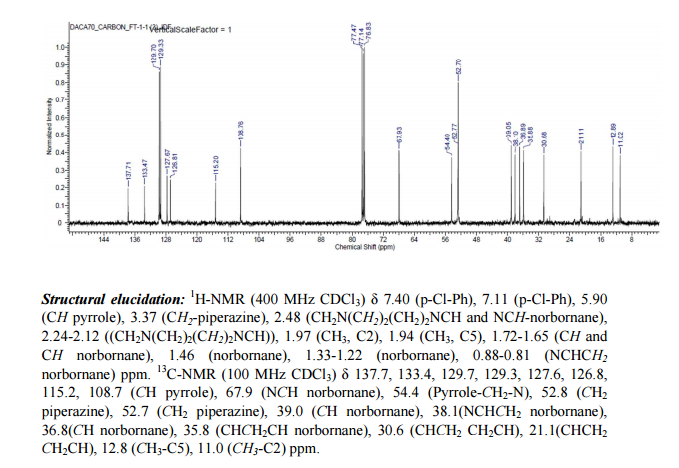
Novel pyrroles have been designed, synthesized, and evaluated against mycobacterial strains. The pyrroles have originally been designed as hybrids of the antitubercular drugs BM212 (1) and SQ109 (2), which showed common chemical features with very similar topological distribution. A perfect superposition of the structures of 1 and 2 revealed by computational studies suggested the introduction of bulky substituents at the terminal portion of the pyrrole C3 side chain and the removal of the C5 aryl moiety. Five compounds showed high activity towardMycobacterium tuberculosis, while 9b and 9c were highly active also against multidrug-resistant clinical isolates. Compound 9c showed low eukaryotic cell toxicity, turning out to be an excellent lead candidate for preclinical trials. In addition, four compounds showed potent inhibition (comparable to that of verapamil) toward the whole-cell drug efflux pump activity of mycobacteria, thus turning out to be promising multidrug-resistance-reversing agents.
Design and Synthesis of 1-((1,5-Bis(4-chlorophenyl)-2-methyl-1H-pyrrol-3-yl)methyl)-4-methylpiperazine (BM212) and N-Adamantan-2-yl-N′-((E)-3,7-dimethylocta-2,6-dienyl)ethane-1,2-diamine (SQ109) Pyrrole Hybrid Derivatives: Discovery of Potent Antitubercular Agents Effective against Multidrug-Resistant Mycobacteria
http://pubs.acs.org/doi/abs/10.1021/acs.jmedchem.6b00031
SQ109 is a drug undergoing development for treatment of tuberculosis.[1][2]
Background
On October 16, 2007, it was given the status of Orphan drug by the U.S. Food and Drug Administration (FDA) for use against drug-susceptible and drug-resistant TB bacteria.[3]
SQ109 completed three phase I studies in the U.S. and one Phase II efficacy studies in tuberculosis patients in Africa. SQ109 showed activity against both drug susceptible and Multi-drug-resistant tuberculosis bacteria, including Extensively drug-resistant tuberculosis strains. In preclinical studies SQ109 enhanced the activity of anti-tubercular drugs isoniazid and rifampin and reduced by >30% the time required to cure mice of experimental TB.
SQ109 is being developed by OOO Infectex in Russia and by Sequella Inc internationally. In July 2012, Infectex received notification from the Russian Ministry of Health for approval to begin the pivotal clinical trial associated with a drug registration submission, and can proceed with the clinical development of SQ109 for treatment of tuberculosis in the Russian Federation.[4]
References
- Jia, L.; Tomaszewski, J. E.; Hanrahan, C.; Coward, L.; Noker, P.; Gorman, G.; Nikonenko, B.; Protopopova, M. (2009). “Pharmacodynamics and pharmacokinetics of SQ109, a new diamine-based antitubercular drug”. British Journal of Pharmacology 144 (1): 80–87. doi:10.1038/sj.bjp.0705984. PMC 1575972. PMID 15644871.
- Meng, Q.; Luo, H.; Liu, Y.; Li, W.; Zhang, W.; Yao, Q. (2009). “Synthesis and evaluation of carbamate prodrugs of SQ109 as antituberculosis agents”. Bioorganic & Medicinal Chemistry Letters 19 (10): 2808–10. doi:10.1016/j.bmcl.2009.03.091. PMID 19362471.
- “New FDA Orphan Drugs: AVI-4658, SQ109, ATIR”. Medscape Medical News. 11 November 2007.
“PRESS RELEASE Maxwell Biotech Venture Fund`s Portfolio Company, Infectex, Receives Russian Regulator`s Approval to Conduct Pivotal Clinical Trial for Sequella’s Antibiotic, SQ109, for Tuberculosis”. Reuters. 26 July 2012.
 |
|
| Names | |
|---|---|
| IUPAC name
N-Adamantan-2-yl-N’-((E)-3,7-dimethyl-octa-2,6-dienyl)-ethane-1,2-diamine
|
|
| Identifiers | |
| 7997 | |
| Jmol 3D model | Interactive image |
| PubChem | 5274428 |
| Properties | |
| C22H38N2 | |
| Molar mass | 330.56 g·mol−1 |
| SQ109; SQ-109; SQ 109; UNII-9AU7XUV31A; CHEMBL561057; N’-(2-adamantyl)-N-[(2E)-3,7-dimethylocta-2,6-dienyl]ethane-1,2-diamine; More… | |
| Molecular Formula: | C22H38N2 |
|---|---|
| Molecular Weight: | 330.55052 g/mol |
SQ109 is an orally active, small molecule antibiotic for treatment of pulmonary TB. Currently in Phase I clinical trials, SQ109 could replace one or more drugs in the current first-line TB drug regimen, simplify therapy, and shorten the TB treatment regimen.
| Patent ID | Date | Patent Title |
|---|---|---|
| US2014045791 | 2014-02-13 | COMBINATION THERAPY TO TREAT MYCOBACTERIUM DISEASES |
| US8202910 | 2012-06-19 | Compositions and methods for treatment of infectious disease |
| US2011118307 | 2011-05-19 | Compositions and methods for the treatment of infectious diseases |
| US7842729 | 2010-11-30 | Anti tubercular drug: compositions and methods |
| US2009281054 | 2009-11-12 | COMPOSITIONS AND METHODS COMPRISING CAPURAMYCIN ANALOGUES |
| US7456222 | 2008-11-25 | Anti tubercular drug: compositions and methods |
| US6951961 | 2005-10-04 | Methods of use and compositions for the diagnosis and treatment of infectious disease |
| US2004033986 | 2004-02-19 | Anti tubercular drug: compositions and methods |
| US2004019117 | 2004-01-29 | Anti tubercular drug: compostions and methods |
///////SQ109, bm 212













Sorry, the comment form is closed at this time.