DS-1040
Daiichi Sankyo Co Ltd
Ischemic stroke
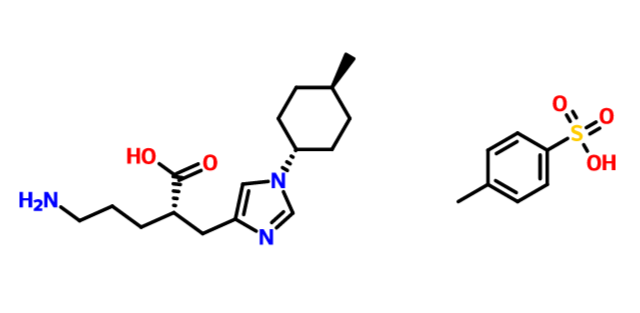
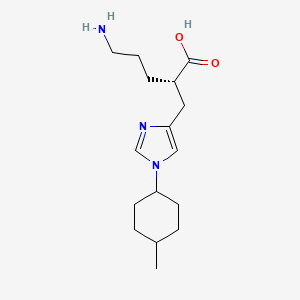
(2S)-5-amino-2-[[1-(4-methylcyclohexyl)imidazol-4-yl]methyl]pentanoic acid
1H-Imidazole-4-propanoic acid, α-(3-aminopropyl)-1-(trans-4-methylcyclohexyl)-, (αS)-
(2S)-5-amino-2-{[1-(trans-4-methylcyclohexyl)-1H-imidazol-4-yl]methyl}pentanoic acid
free form cas 1335138-62-9
1:1 TOSYLATE 1335138-89-0
1335138-90-3 1:1:1 TOSYLATE HYDRATE
phase 2, Ischemic stroke
| Molecular Formula: |
C16H27N3O2 |
| Molecular Weight: |
293.40448 g/mol |

TAFIa inhibitors, useful for treating myocardial infarction, angina, pulmonary hypertension and deep vein thrombosis.
In March 2016, DS-1040 was reported to be in phase 2 C clinical development, and the study was expected to complete in June 2017.
https://clinicaltrials.gov/ct2/show/NCT02560688
- 01 Feb 2016Daiichi Sankyo initiates a phase I trial in Healthy volunteers in United Kingdom (NCT02647307)
- 09 Jan 2016Daiichi Sankyo plans a phase I trial in Healthy volunteers in United Kingdom (NCT02647307)
- 29 Sep 2015Daiichi Sankyo plans a drug-interaction phase I trial in Healthy volunteers in United Kingdom (IV) (NCT02560688)
SYNTHESIS
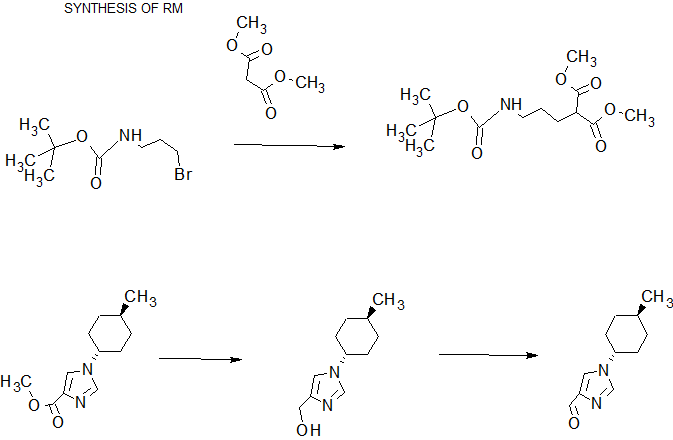
COMPLETE SYNTHESIS
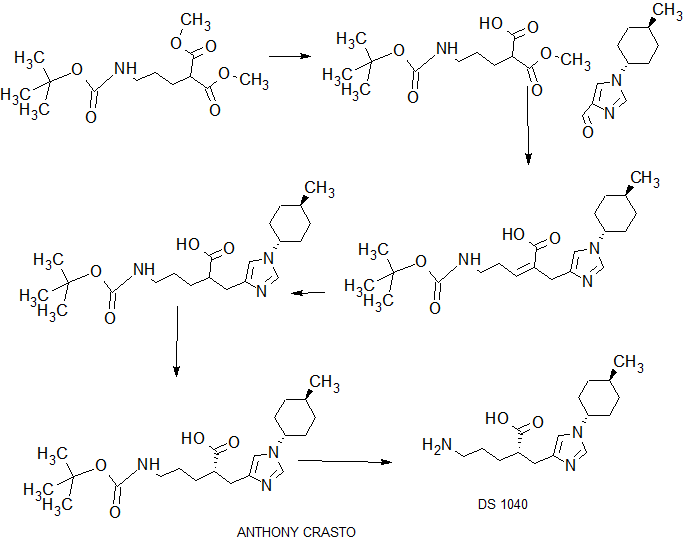
WO201111506
WO2013039202
WO 2016043254
PATENT
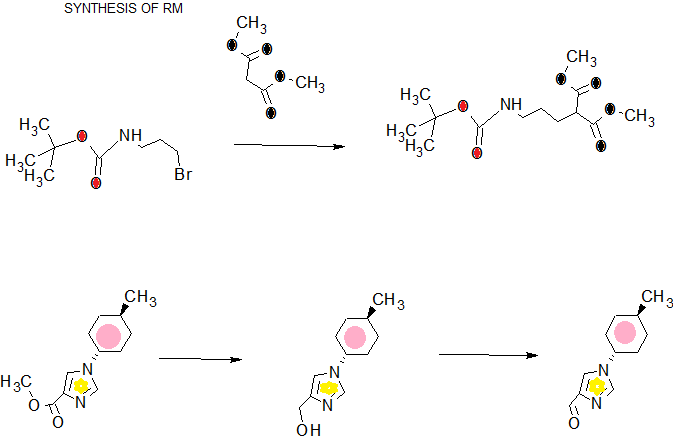
COMPLETE SYN……….
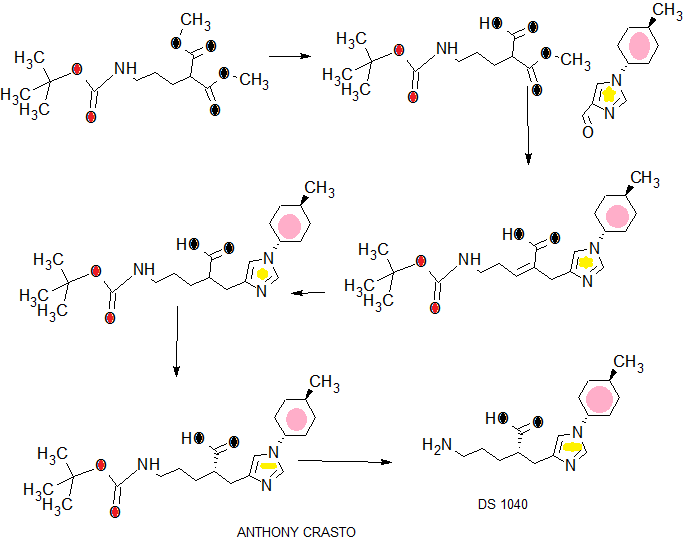
WO2016043253


The optical purity of the obtained compound was measured by the following HPLC analysis conditions.
(2S) -5 – [(tert- butoxycarbonyl) amino] -2 – {[1- (trans -4- methylcyclohexyl)-lH-imidazol-4-yl] methyl} valeric acid (S)-2-amino 1-propanol salt (A1 step, A2 step, A3 step), (2S) -5 – [ (tert- butoxycarbonyl) amino] -2 – {[1- (trans -4- methylcyclohexyl)-lH-imidazole 4-yl] methyl} optical purity measurement conditions valerate (A4 step):
column: CHIRAL AGP 4.6mmI. D. × 250mm (5μm),
mobile phase: methanol / 10mM phosphate buffer solution (pH7.0) = 95/5,
temperature: 40 ℃,
flow rate: 0.5mL / min,
detection method: UV at 220nm,
retention time: R body: 5.9 minutes, S body: 7.3 minutes.
(2S)-5-amino-2 – Optical purity measurement conditions {[1- (trans-4- methylcyclohexyl)-lH-imidazol-4-yl] methyl} valerate p- toluenesulfonate (A5 Step) :
column: CHIRLCEL OZ-H 4.6mmI. D. × 250mm (5μm),
mobile phase: hexane / ethanol / methanol / isopropanol / trifluoroacetic acid / triethylamine = 860/100/20/2/2
temperature: 30 ℃
flow rate: 1.0mL / min
detection method: UV at 220nm
retention time: R body: 16.1 minutes, S body: 13.0 minutes (example 1) (1-1) 5 – [(Tert- butoxycarbonyl) amino] -2-methoxy-carbonyl) valeric acid morpholine salt
[Of 11]

In methanol (400mL) solution of di -tert- butyl (100.0g) and 3-chloro-propylamine hydrochloride (71.5g), was added dropwise triethylamine (51.0g) at 0 ℃, at the same temperature It was stirred for 16 hours. To the reaction solution was added toluene (400 mL) and water (400 mL), then were separated, and the organic layer was washed with water. Toluene 400mL was added to the organic layer, was concentrated under reduced pressure to 300 mL, N, N-dimethylacetamide (210 mL) was added and concentrated in vacuo to 300 mL. Potassium carbonate solution (126.66g), tetrabutylammonium bromide (44.32g), was added dimethyl malonate (90.82g) and N, N-dimethylacetamide (100 mL), stirred for 20 hours at 55 ° C. did. Toluene (400 mL) and water (700 mL) was added to the reaction mixture, after separation, The organic layer was washed with water, with 1M aqueous sodium hydroxide and water, and concentrated under reduced pressure to 150 mL. This solution methanol (1870mL) and 1M sodium hydroxide solution (430.8mL) in addition to, and the mixture was stirred for 27.5 hours at 0 ℃. Concentrated hydrochloric acid to the reaction solution (2.5 mL) was added, the pH was adjusted to 7-9, and concentrated in vacuo to 375 mL. After addition of ethyl acetate (500mL) to the reaction solution, concentrated hydrochloric acid (35.1mL) was added, the pH was adjusted to 2.2-2.5, and the layers were separated. The aqueous layer was extracted with ethyl acetate (500 mL), after mixing the organic layer under reduced pressure, and prepared by dehydration condensation of ethyl acetate (250 mL) solution. The resulting solution of ethyl acetate (500 mL) and morpholine (37.5 g) was added to and stirred overnight. The precipitated crystals were filtered, washed with ethyl acetate, and dried under reduced pressure, to give the title compound (136.1g, 81.9% yield).
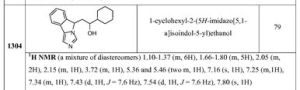
1 H-NMR (DMSO-d- . 6 ) [delta]: 6.79 (1H, t, J = 5.5 Hz), 3.61 (4H, t, J = 4.9 Hz), 3.58 The (3H, s) , 3.14 (1H, t, J = 7.8Hz), 2.90-2.80 (6H, m), 1.74-1.59 (2H, m), 1.37 (9H, s) , 1.34-1.25 (2H, m).
(1-2) [1- (trans-4- methylcyclohexyl) -1H- imidazole-4-yl] methanol
[Of 12]

N, and stirred for 4 h methanol (56 mL) solution at 5 ~ 10 ℃ of N- dimethylformamide dimethyl acetal (77.4 g) and ethyl isocyanoacetate (70.0g).The reaction solution was cooled to 0 ℃, water (5.3mL) and trans-4- methylcyclohexyl amine (105.1g) was added, and the mixture was stirred for 24 hours at 60 ~ 65 ℃. The reaction was cooled to room temperature, toluene (420 mL), supplemented with 10% brine (280 mL) and concentrated hydrochloric acid (68 mL), After separation, the organic layer was washed with 10% brine (140 mL). Organic layer to 10% sodium chloride solution (280mL) and concentrated hydrochloric acid were added for liquid separation after (78.4g), was added to separate liquid further 10% saline solution into the organic layer (210mL) and concentrated hydrochloric acid (31.3g). After dissolving sodium chloride (70.0 g) in aqueous layer, adding toluene (420 mL) and 50% aqueous sodium hydroxide (85 mL), after separation, toluene (350 mL) the organic layer was added, under reduced pressure, dehydration concentrated was prepared in toluene (420 mL) solution was. The solution was cooled to 0 ℃, dropped the hydrogenated bis (2-methoxyethoxy) aluminum sodium (70% toluene solution) (207.4g), and the mixture was stirred at room temperature for 1 hour. The reaction was cooled to 0 ° C., was added dropwise 12.5% aqueous sodium hydroxide solution (700 mL), stirred for 1 hour at room temperature. After the solution was separated and the organic layer was washed successively with 12.5% aqueous solution of sodium hydroxide (700mL) and 20% sodium chloride solution (140mL), toluene in the organic layer (140mL), 1- butanol (14mL), water ( 280mL) and was added to aliquots of concentrated hydrochloric acid (48mL). It was further added to liquid separation with water (140 mL) and concentrated hydrochloric acid (2 mL) to the organic layer. Met The aqueous layer was stirred in for 1 hour activated carbon (10.5 g), activated charcoal was filtered off, the activated carbon was washed with water (210 mL). Matches the filtrate and washings, sodium chloride (140 g), toluene was added (980 mL) and 50% aqueous sodium hydroxide (42 mL), After separation, under reduced pressure and the organic layer was dried concentrated toluene (210 mL) It was prepared in solution. The solution was stirred 30 minutes at 50-55 ° C., cooled to room temperature, it was added dropwise heptane (560 mL), and stirred at the same temperature for 3 hours. The precipitated crystals were filtered to give after washing with toluene / heptane (1/4) mixture solution, the title compound was dried under reduced pressure (77.2 g, 64.2% yield).
1 H-NMR (CDCl 3 ) [delta]: 7.49 (1H, s), 6.91 (1H, s), 4.58 (2H, s), 3.83 (1H, tt, J = 12.0 , 3.9Hz), 2.10-2.07 (2H, m), 1.87-1.84 (2H, m), 1.70-1.61 (2H, m), 1.48-1 .42 (1H, m), 1.15-1.06 (2H, m), 0.95 (3H, d, J = 6.5Hz).
(1-3) (2E) -5 – [(tert- butoxycarbonyl) amino] -2 – {[1-trans-4- methylcyclohexyl]-lH-imidazol-4-yl} methylidene} methyl valerate
[Of 13]

(1-2) The compound obtained in (50.0 g) in toluene (350 mL) and acetic acid (150 mL) was dissolved in a mixed solution, 2,2,6,6-tetramethylpiperidine -N- oxyl at 30 ° C. It was added (966mg) and ortho-periodic acid (16.9g), and the mixture was stirred for 1 hour at 30-35 ℃. The reaction mixture was added 10% aqueous sodium bisulfite solution (150 mL), after stirring for 30 minutes at room temperature, toluene was added (400 mL), and concentrated in vacuo to 300 mL. The solution further by the addition of toluene (400 mL), after concentration under reduced pressure again to 300 mL, was added toluene (500 mL), water (200 mL) and 50% aqueous sodium hydroxide (118 mL). Were separated, the organic layer was washed with 20% brine (150 mL), addition of toluene (200 mL), under reduced pressure and dehydrated concentrated prepared in toluene (400 mL) solution. The compound obtained in the solution (1-1) (116.5g), N, N- dimethylformamide (175 mL) and acetic acid (4.2 mL) was added, under reduced pressure, and dried for 8 hours under reflux. The reaction was cooled to room temperature, adding toluene (400 mL), washed once with 3 times with 5% aqueous sodium bicarbonate solution (400 mL) and 10% brine (250 mL), under reduced pressure and the organic layer was dried concentrated toluene It was prepared (900 mL) solution. This solution was added activated charcoal (15 g) at 35 ~ 40 ° C., after stirring for 30 minutes at the same temperature, filtered and the activated carbon was washed with toluene. Meet the filtrate and washings, after which was concentrated under reduced pressure until 250mL, it was added dropwise heptane (500mL) at room temperature. After stirring for 1.5 hours at the same temperature, then cooled to 0 ℃, and the mixture was stirred for 1 hour. The precipitated crystals were filtered to give after washing with toluene / heptane (1/2) mixture solution, the title compound was dried under reduced pressure (85.0 g, 81.5% yield).
1 H-NMR (CDCl 3 ) [delta]: 7.59 (1H, s), 7.47 (1H, s), 7.15 (1H, s), 7.08 (1H, brs), 3.92- 3.87 (1H, m), 3.78 (3H, s), 3.16-3.12 (2H, m), 2.96 (2H, t, J = 7.5Hz), 2.14- 2.11 (2H, m), 1.90-1.87 (2H, m), 1.77-1.65 (5H, m), 1.47 (9H, s), 1.17-1. 10 (2H, m), 0.96 (3H, d, J = 6.5Hz).
(1-4) (2S) -5 – [(tert- butoxycarbonyl) amino] -2 – {[1- (trans-4- methylcyclohexyl)-lH-imidazol-4-yl] methyl} valerate (S ) -2-amino-1-propanol salt (A1 process, A2 process, A3 process)
[Of 14]

The compound obtained in (1-3) (40.0g), (R) -2,2′- bis (di-3,5-xylyl) -1,1′-binaphthyl (507.4Mg) and dichloro (p- cymene) ruthenium (II) (dimer) and (211.4mg), were dissolved in degassed 2,2,2 trifluoroethanol (400 mL), hydrogen under pressure (400-450kPa) , and the mixture was stirred for 24 hours at 60 ℃. The reaction was cooled to room temperature, after nitrogen substitution, and then concentrated under reduced pressure to 60 mL.Tetrahydrofuran (200 mL) was added, was concentrated under reduced pressure to 120 mL, of tetrahydrofuran was added (200 mL).
To the resulting solution was added water (160mL), cooled to 0 ℃, was added a 50% aqueous solution of sodium hydroxide (24.0mL). After stirring the reaction mixture at room temperature for 26 hours, and the addition of 50% sodium hydroxide solution (8.00mL), and the mixture was stirred for a further 4 hours. The reaction mixture under ice-cooling was added dropwise concentrated hydrochloric acid (28 mL), activated carbon was added (2.0 g) was stirred at room temperature for 10 minutes. The active carbon was filtered off, washed with tetrahydrofuran / water (2/1) mixed solvent (180 mL), sodium chloride (40 g) was separated by adding and re-extract the aqueous layer with tetrahydrofuran (400 mL). The organic layer was matched, and concentrated in vacuo to 200 mL. After addition of toluene (400 mL) to this solution, under reduced pressure and dehydrated concentrated prepared in toluene (200 mL) solution.
After adding tetrahydrofuran (400 mL) to the resulting solution was added (S) -2- amino-1-propanol (8.2 g) at room temperature and stirred for 3 hours. The solution was cooled to 0 ℃, and was filtered after stirring for 1.5 hours, it was precipitated crystals. The crystals were washed with tetrahydrofuran and dried under reduced pressure to give the title compound (45.4g, 98.2% yield, optical purity: ee 97.5%) was obtained.
1 H-NMR (CD 3 OD) [delta]: 7.57 (1H, s), 6.94 (1H, s), 3.98-3.85 (1H, yd), 3.69-3.64 ( 1H, m), 3.47-3.42 (1H , m), 3.29-3.23 (1H, m), 3.01 (2H, t, J = 6.5Hz), 2.84 ( 1H, dd, J = 14.6,8.4Hz) , 2.55 (1H, dd, J = 14.6,6.2Hz), 2.52-2.45 (1H, m), 2.03 (2H, d, J = 12.7Hz ), 1.83 (2H, d, J = 13.3Hz), 1.71 (2H, q, J = 12.5Hz), 1.60-1.44 ( 5H, m), 1.41 (9H , s), 1.23-1.20 (3H, m), 1.18-1.09 (2H, m), 0.94 (3H, d, J = 6.8Hz).
(1-5) (2S) -5 – [(tert- butoxycarbonyl) amino] -2 – {[1- (trans-4- methylcyclohexyl)-lH-imidazol-4-yl] methyl} valerate (A4 process)
[Of 15]

(1-4) The compound obtained in (40.0 g) in tetrahydrofuran (400 mL) and dissolved in a mixed solvent of water (160 mL), concentrated hydrochloric acid (7.3 mL) and added separation of sodium chloride (40 g) and washed 3 times with the organic layer 20% (w / w) brine (160 mL). The organic layer under reduced pressure, dehydrated concentrated prepared in toluene (320 mL) solution was dissolved after addition of tetrahydrofuran (80 mL) was warmed precipitated 83 ° C. crystal. After stirring overnight and cooled to room temperature, and stirred for a further 3 hours at 0 ℃, and filtered the precipitated crystals. After washing the crystals with toluene / tetrahydrofuran (4/1) mixed solution, and dried under reduced pressure to give the title compound (30.9g, 92.1% yield, optical purity: 97.4% ee) was obtained.
1 H-NMR (CDCl 3 ) [delta]: 7.59 (1H, s), 6.73 (1H, s), 4.67 (1H, brs), 3.85-3.80 (1H, yd), 3.12-3.08 (2H, m), 2.88 (1H, dd, J = 15.2,8.8Hz), 2.79 (1H, dd, J = 15.2,3.6Hz) , 2.70-2.64 (1H, m), 2.13-2.06 (2H, m), 1.90-1.82 (2H, m), 1.79-1.52 (5H, m), 1.49-1.44 (2H, m ), 1.43 (9H, s), 1.15-1.05 (2H, m), 0.95 (3H, d, J = 6. 5Hz).
(1-6) (2S) -5- amino -2 – {[1- (trans-4- methylcyclohexyl)-lH-imidazol-4-yl] methyl} valerate p- toluenesulfonate (A5 Step)
[Of 16]

In tetrahydrofuran (100 mL), was dissolved the compound obtained in (1-5) (25.0 g) and p- toluenesulfonic acid monohydrate (13.3 g), activated charcoal (1 to this solution. 25 g) was added and stirred for 1 hour at 20 ~ 30 ℃. The charcoal was filtered and washed with tetrahydrofuran (50 mL).It matches the filtrate and washings, p- toluenesulfonic acid monohydrate (13.3 g) and water (7.5 mL) and the mixture was heated under reflux for 6 hours. The reaction was cooled to room temperature, it was added triethylamine (7.7 g), at room temperature and stirred overnight. To the reaction solution was added dropwise tetrahydrofuran (350 mL), after stirring for 3 hours at room temperature and filtered the precipitated crystal. After washing with tetrahydrofuran / water (50/1) mixed solution, and dried under reduced pressure to give the title compound (27.7g, 93.5% yield, optical purity: 98.4% ee) was obtained.
1 H-NMR (CD 3 OD) [delta]: 8.18 (1H, s), 7.70 (2H, d-, J = 8.1 Hz), 7.22 (2H, d-, J = 7.5 Hz), 7.16 (1H, s), 4.06 (1H, tt, J = 12.0,3.9Hz), 2.94-2.86 (3H, m), 2.69 (1H, dd, J = 14.6,5.8Hz), 2.62-2.59 (1H, m), 2.36 (3H, s), 2.08-2.05 (2H, m), 1.86-1 .83 (2H, m), 1.76-1.46 (7H, m), 1.18-1.11 (2H, m), 0.94 (3H, d, J = 6.5Hz).
(Example
2) (2-1) (2S) -5 – [(tert-butoxycarbonyl) amino] -2 – {[1- (trans -4- methylcyclohexyl)-lH-imidazol-4-yl] methyl } methyl valerate
[Of 17]

It was asymmetrically reduced using a number of catalysts. The reaction conversion and the optical purity of the obtained title compound was determined by the following HPLC analysis conditions.
Reaction conversion rate measurement:
Column: Waters XBridge C18 4.6mmI. D. × 150mm (3.5μm),
mobile phase: (A) 10mM aqueous ammonium acetate solution, (B)
acetonitrile, Gradient conditions: B: conc. ; 20% (0-5 minutes), 20-90% (5-20 minutes), 90% (20-24 minutes),
temperature: 40 ℃,
flow rate: 1.0mL / min,
detection method: UV at 215nm
retention time: raw material: 21.1 minutes, the product: 19.1 minutes,
(peak area of peak area + product of raw materials) peak area / of the reaction conversion rate = product.
Optical purity measurement conditions:
column: CHIRALPAK IA 4.6mmI. D. × 250mm (5μm),
mobile phase: ethanol / hexane = 20/80
Temperature: 35 ℃,
flow rate: 1.0mL / min,
detection method: UV at 210nm,
retention time: R body: 6.8 minutes, S body: 7.8 minutes.
PATENT
Daiichi Sankyo Company,Limited, 第一三共株式会社
WO2011115064…..
http://www.google.co.in/patents/WO2011115064A1?cl=en
[Reference Example 1] 5 – [(tert- butoxycarbonyl) amino] -2- (diethoxyphosphoryl) valeric acid tert- butyl

Diethylphosphonoacetate tert- butyl (20.0g) was dissolved in tetrahydrofuran (500mL), sodium hydride (63%, 3.32g) was added at 0 ℃, 15 min at 0 ℃, and stirred for 1 hour at room temperature . (3-bromopropyl) tetrahydrofuran carbamic acid tert- butyl (20.0g) (20mL) was slowly at room temperature, and the mixture was stirred at room temperature for 18 hours. A saturated aqueous solution of ammonium chloride was added to the reaction solution, the organic matter was extracted with ethyl acetate. The organic layer was washed with saturated brine, dried over anhydrous sodium sulfate, and filtered to give the solvent was distilled off under reduced pressure the crude product. This silica gel column chromatography and purified (eluent hexane / ethyl acetate = 1/1-ethyl acetate) to give the title compound (26.6g).
1 H-NMR (CDCl 3) δ: 1.31-1.36 (6H, m), 1.44 (9H, m), 1.48 (9H, m), 1.51-1.59 (2H, m), 1.78-2.00 (2H, m) , 2.83 (1H, ddd, J = 22.9, 10.7, 4.4 Hz), 3.06-3.18 (2H, m), 4.10-4.18 (4H, m), 4.58 (1H, br).
[Reference Example 2] 5 – [(tert- butoxycarbonyl) amino] -2- (1H- imidazol-4-ylmethyl) valeric acid tert- butyl

In acetonitrile (100mL) solution of the compound obtained in Reference Example 1 (8.35g), at room temperature 1,8-diazabicyclo [5.4.0] undec-7-ene (4.58mL) and lithium chloride (1 .30g) and we were added. The suspension was added with 1-trityl–1H- imidazole-4-carbaldehyde (6.90g) was stirred at room temperature overnight, under vacuum, and the solvent was evaporated. After the solution separated by adding ethyl acetate and 10% citric acid aqueous solution, an organic layer, saturated brine, and then washed with a saturated aqueous sodium bicarbonate solution and brine. Dried over anhydrous sodium sulfate, (2E) -5 – [(tert- butoxycarbonyl) amino] -2 – [(1-trityl–1H- imidazol-4-yl) methylene] valeric acid tert- butyl and (2Z) -5 – obtain [(1-trityl–1H- imidazol-4-yl) methylene] valeric acid tert- butyl mixture (11.3g) – [(tert- butoxycarbonyl) amino] -2. The mixture was suspended in methanol (500mL), 10% palladium-carbon catalyst (water content, 4g) was added and stirred for 3 days at room temperature under hydrogen atmosphere. The catalyst was removed by filtration, and the filtrate was concentrated under reduced pressure. Silica gel chromatography gave (eluting solvent: methylene chloride / methanol = 9/1) the title compound (5.60g).
1 H-NMR (CDCl 3) δ: 1.41 (9H, s), 1.44 (9H, s), 1.48-1.57 (3H, m), 1.57-1.66 (1H, m), 2.58-2.68 (1H, m) , 2.73 (1H, dd, J = 14.7, 5.3 Hz), 2.89 (1H, dd, J = 14.7, 8.4 Hz), 3.02-3.19 (2H, m), 4.67 (1H, br s), 6.79 (1H, s), 7.54 (1H, s).
[Reference Example 3] 5 – [(tert- butoxycarbonyl) amino] -2- (methoxycarbonyl) valeric acid

Sodium methoxide in dimethyl malonate (102mL) – methanol (28%, 90.4mL) was added at room temperature and stirred at 60 ℃ 30 minutes. After cooling the white suspension solution to room temperature, (3-bromopropyl) was added carbamic acid tert- butyl (106g) in one portion and stirred at room temperature for 12 hours. Water was added to the reaction solution and the organics extracted with diethyl ether. The organic layer was successively washed with 1 N sodium hydroxide aqueous solution and saturated brine, dried over anhydrous sodium sulfate, filtered and the solvent was distilled off under reduced pressure {3 – [(tert- butoxycarbonyl) amino] propyl} malonic I got acid dimethyl of crude product. The resulting ester (94g) was dissolved in methanol (100mL), water lithium hydroxide monohydrate (13.6g) (300mL) – was added to methanol (300mL) solution at 0 ℃, 15 h stirring at room temperature It was. The methanol was distilled off under reduced pressure and the organics were extracted with ethyl acetate. 2N hydrochloric acid (160mL) was added to the aqueous layer was extracted with ethyl acetate. The organic layer was washed with saturated brine, dried over anhydrous sodium sulfate, and filtered to give the solvent was distilled off under reduced pressure the crude product. This silica gel column chromatography: – is purified (eluent methylene chloride methylene chloride / methanol = 10/1) to give the title compound (69.1g).
1 H-NMR (CDCl 3) δ: 1.44 (9H, m), 1.50-1.60 (2H, m), 1.86-2.01 (2H, m), 3.07-3.20 (2H, m), 3.43 (1H, m) , 3.77 (3H, s), 4.64 (1H, br).
[Reference Example 4] 1- (trans-4- methylcyclohexyl) -1H- imidazole-4-carbaldehyde [Step 1] 1- (trans-4- methylcyclohexyl) -1H- imidazole-4-carboxylic acid ethyl

Was dissolved in 3- (dimethylamino) -2-isocyanoethyl ethyl acrylic acid (Liebigs Annalen der Chemie, 1979 years 1444 pages) (1.52g) and the trans-4- methyl cyclohexylamine (3.07g), 70 ℃ in it was stirred for 4 hours. A saturated aqueous solution of ammonium chloride was added to the reaction solution, the organic matter was extracted with ethyl acetate. The organic layer was dried over anhydrous sodium sulfate, and filtered to give the solvent was distilled off under reduced pressure the crude product. This silica gel column chromatography and purified (eluent hexane / ethyl acetate = 2 / 1-1 / 2) to give the title compound (1.90g).
1 H-NMR (CDCl 3) δ: 0.96 (3H, d, J = 6.6 Hz), 1.13 (2H, m), 1.39 (3H, d, J = 7.0 Hz), 1.47 (1H, m), 1.68 ( 2H, m), 1.88 (2H, m), 2.12 (2H, m), 3.91 (1H, tt, J = 12.1, 3.9 Hz), 4.36 (2H, q, J = 7.0 Hz), 7.54 (1H, s ), 7.66 (1H, s).
[Step 2] [1- (trans-4- methylcyclohexyl) -1H- imidazole-4-yl] methanol
Lithium aluminum hydride (92%, 0.31g) it was suspended in tetrahydrofuran (6mL). The compound obtained in Step 1 of this reference example (1.50g) was dissolved in tetrahydrofuran (6mL), it was slowly added dropwise to the suspension at 0 ℃.0 After stirring for 30 min at ℃, the reaction solution was diluted with diethyl ether, it was added a saturated aqueous solution of sodium sulfate. After stirring for 1 hour at room temperature, the resulting inorganic salt was removed by filtration through Celite. The filtrate to give the crude product was concentrated under reduced pressure. Mixed solvent of this from hexane and ethyl acetate: water (5 1), to give the title compound (1.09g).
1 H-NMR (CDCl 3) δ: 0.95 (3H, d, J = 6.6 Hz), 1.04-1.17 (2H, m), 1.44 (1H, m), 1.59-1.73 (2H, m), 1.81-1.89 (2H, m), 2.04-2.13 (2H, m), 2.78 (1H, br), 3.84 (1H, tt, J = 12.1, 3.9 Hz), 4.59 (2H, s), 6.91 (1H, s), 7.49 (1H, s).
[Step 3] 1- (trans-4- methylcyclohexyl) -1H- imidazole-4-carbaldehyde
The compound obtained in Step 2 of this reference example (1.04g) was dissolved in toluene (10mL). Aqueous solution of sodium hydrogen carbonate (1.35g) (5mL), iodine (2.72g) and 2,2,6,6-tetramethyl-1-sequential piperidinyloxy (84mg) was added and stirred for 2 hours at room temperature It was. The reaction solution was added saturated aqueous sodium thiosulfate solution and the organics were extracted with ethyl acetate. The organic layer was dried over anhydrous sodium sulfate, and filtered to give the solvent was distilled off under reduced pressure the crude product. This silica gel column chromatography and purified (eluent hexane / ethyl acetate = 1 / 1-1 / 2) to give the title compound (0.900g).
1 H-NMR (CDCl 3) δ: 0.97 (3H, d, J = 6.8 Hz), 1.09-1.19 (2H, m), 1.48 (1H, m), 1.65-1.75 (2H, m), 1.87-1.93 (2H, m), 2.11-2.18 (2H, m), 3.95 (1H, tt, J = 12.2, 3.9 Hz), 7.62 (1H, s), 7.68 (1H, s), 9.87 (1H, s).
[Example 15] (2R) -5- amino -2 – {[1- (trans-4- methylcyclohexyl) -1H- imidazole-4-yl] methyl} valeric acid and (2S) -5- amino-2 – {[1- (trans-4- methylcyclohexyl) -1H- imidazol-4-yl] methyl} valeric acid [Step 1] 5 – [(tert- butoxycarbonyl) amino] -2 – {[1- (trans 4-methylcyclohexyl) -1H- imidazole-4-yl] methyl} methyl valerate

The compound obtained in Reference Example 4 (300mg) and the compound obtained in Reference Example 3 (860mg) was suspended in cyclohexane (10mL). Piperidine (0.154mL) and cyclohexane propionic acid (0.116mL) and (10mL) solution was added, and the mixture was heated under reflux for 48 hours. After cooling, aqueous potassium carbonate solution was added to the reaction solution, and the organic matter was extracted with ethyl acetate. The organic layer was washed with saturated brine, dried over anhydrous sodium sulfate, filtered, and the solvent was evaporated under reduced pressure. The obtained crude product was dissolved in ethanol (12mL), 10% palladium-carbon catalyst (water, 250mg) was added and atmospheric pressure hydrogen atmosphere at room temperature for 4 hours and stirred at 60 ℃ 2.5 hours. After Celite filtration, to give the crude product and the filtrate was concentrated under reduced pressure. This silica gel column chromatography and purified (eluent hexane / ethyl acetate = 2 / 1-1 / 3) to give the title compound (562mg).
1 H-NMR (CDCl 3) δ: 0.94 (3H, d, J = 6.6 Hz), 1.02-1.15 (2H, m), 1.34-1.69 (7H, m), 1.43 (9H, s), 1.80-1.87 (2H, m), 1.99-2.09 (2H, m), 2.69 (1H, dd, J = 13.7, 6.3 Hz), 2.79 (1H, m), 2.88 (1H, dd, J = 13.7, 7.4 Hz), 3.03-3.13 (2H, m), 3.63 (3H, s), 3.79 (1H, tt, J = 12.1, 3.9 Hz), 4.76 (1H, br), 6.67 (1H, s), 7.47 (1H, s) .
[Step 2] (2R) -5 – [(tert- butoxycarbonyl) amino] -2 – {[1- (trans-4- methylcyclohexyl) -1H- imidazol-4-yl] methyl} methyl valerate and ( 2S) -5 – [(tert- butoxycarbonyl) amino] -2 – {[1- (trans-4- methylcyclohexyl) -1H- imidazol-4-yl] methyl} methyl valerate
The compound obtained in Step 1 of this Example (40mg) was dissolved in hexane (1.5mL) and ethanol (0.5mL), using CHIRALPAK IA semi-preparative column (2.0cm × 25.0cm) It was optically resolved by high performance liquid chromatography. Flow rate: 15mL / min, elution solvent: hexane / ethanol = 75/25, detection wavelength: 220nm.
The solvent of the divided solution was evaporated under reduced pressure to give both enantiomers each (15mg). Both enantiomers were confirmed to be optically pure by analytical HPLC. Column: CHIRALPAK IA (0.46cm × 25.0cm), flow rate: 1mL / min, elution solvent: hexane / ethanol = 80/20 <v / v>, detection wavelength: 220nm, retention time: (2R) -5- [(tert- butoxycarbonyl) amino] -2 – {[1- (trans-4- methylcyclohexyl) -1H- imidazol-4-yl] methyl} methyl valerate (7.2 min), (2S) -5 – [(tert- butoxycarbonyl) amino] -2 – {[1- (trans-4- methylcyclohexyl) -1H- imidazol-4-yl] methyl} methyl valerate (11.2 min).
[Step 3] (2R) -5- amino -2 – {[1- (trans-4- methylcyclohexyl) -1H- imidazole-4-yl] methyl} valerate
Obtained in Step 2 of this Example (2R) -5 – [(tert- butoxycarbonyl) amino] -2 – {[1- (trans-4- methylcyclohexyl) -1H- imidazol-4-yl] methyl } the methyl valerate (15.0mg) was added to 5 N hydrochloric acid (2mL), and the mixture was heated under reflux for 4 hours. After cooling, the solvent it was evaporated under reduced pressure. The resulting crude hydrochloride salt was dissolved in methanol, was added DOWEX50WX8-200. After the resin was washed with water and eluted with 4% aqueous ammonia. The eluate was concentrated, the crude product was washed with acetone to give the title compound (2.2mg).
[Step 4] (2S) -5- amino -2 – {[1- (trans-4- methylcyclohexyl) -1H- imidazole-4-yl] methyl} valerate
Obtained in Step 2 of this Example (2S) -5 – [(tert- butoxycarbonyl) amino] -2 – {[1- (trans-4- methylcyclohexyl) -1H- imidazol-4-yl] methyl } the methyl valerate (15.0mg) was added to 5 N hydrochloric acid (2mL), and the mixture was heated under reflux for 4 hours. After cooling, the solvent it was evaporated under reduced pressure. The resulting crude hydrochloride salt was dissolved in methanol, was added DOWEX50WX8-200 (200mg). After the resin was washed with water, ammonia water (4%, 80mL) and eluted with. The eluate was concentrated, the crude product was washed with acetone to give the title compound (1.8mg).
[Example 16] 5-amino -2 – {[1- (trans-4- methylcyclohexyl) -1H- imidazole-4-yl] methyl} valeric acid benzyl hydrochloride [Step 1] 5 – [(tert- butoxycarbonyl) amino] -2 – {[1- (trans-4- methylcyclohexyl) -1H- imidazol-4-yl] methyl} valerate
The compound obtained in step 1 of Example 15 (7.00g) was dissolved in a mixed solvent consisting of tetrahydrofuran (70mL) and water (14mL), lithium hydroxide monohydrate and (1.26g) at room temperature The mixture was stirred overnight.The reaction solution 2 N hydrochloric acid (8.6mL) was added to neutralize, followed by distilling off the solvent under reduced pressure. The resulting residue was dried with anhydrous sodium sulfate added methylene chloride was to give the crude product was distilled off the solvent under reduced pressure the title compound. This it was used in the next reaction.
MS (ESI) m / z 394 [M + H] +.
[Example 40] (2S) -5- Amino -2 – {[1- (trans-4- methylcyclohexyl) -1H- imidazol-4-yl] methyl} valerate · p- toluenesulfonate, anhydrous
The compound obtained in Step 4 of Example 15 (2.04g) was suspended stirring in tetrahydrofuran (15mL), p- toluenesulfonate monohydrate (1.32g) was added, at room temperature for 1 day the mixture was stirred. The precipitated crystals were collected by vacuum filtration to obtain dried in one day like the title compound (3.01g).
1 H-NMR (CD 3 OD) δ: 0.95 (3H, d, J = 6.5 Hz), 1.11-1.21 (2H, m), 1.43-1.79 (7H, m), 1.83-1.89 (2H, m), 2.05-2.10 (2H, m), 2.37 (3H, s), 2.57-2.64 (1H, m), 2.70 (1H, dd, J = 14.5, 5.5 Hz), 2.85-2.95 (3H, m), 4.07 ( 1H, tt, J = 11.7, 3.9 Hz), 7.18 (1H, s), 7.23 (2H, d, J = 7.8 Hz), 7.70 (2H, d, J = 8.2 Hz), 8.22 (1H, s).
Elemental analysis: C 16 H 27 N 3 O 2 · C 7 H 8 O 3 S,
Theoretical value: C; 59.33, H; 7.58, N; 9.02, O; 17.18, S; 6.89,
Measured value: C; 59.09, H; 7.53, N; 8.92, O; 17.22, S; 6.78.
———————————-.
[Example 41] (2S) -5- Amino -2 – {[1- (trans-4- methylcyclohexyl) -1H- imidazol-4-yl] methyl} valerate · p- toluenesulfonate & 1 Water hydrate
The obtained compound (101.6mg) in 6% water-containing tetrahydrofuran (600μL) was added in Example 40, was dissolved by heating at 60 ℃. Was allowed to stand at room temperature for 1 day, it was collected by filtration and the precipitated crystals were obtained by dried for one day wind the title compound (79.3mg).
Elemental analysis: C 16 H 27 N 3 O 2 · C 7 H 8 O 3 S · 1H 2 O,
Theoretical value: C; 57.12, H; 7.71, N; 8.69, O; 19.85, S; 6.63,
Measured value: C; 56.90, H; 7.69, N; 8.67, O; 19.81, S; 6.42.
References
Study to Assess the Safety, Pharmacokinetics, and Pharmacodynamics of DS-1040b in Subjects With Acute Ischemic Stroke (NCT02586233
Phase I Study to Evaluate the Safety and Tolerability of DS-1040b Intravenous Infusion With Clopidogrel in Healthy Subjects (NCT02560688)
Study of the Effects of Ethnicity on the Pharmacokinetics, Pharmacodynamics and Safety of DS-1040b (NCT02647307)
Edo, N.; Noguchi, K.; Ito, Y.; Morishima, Y.; Yamaguchi, K.
Hemorrhagic risk assessment of DS-1040 in a cerebral ischemia/reperfusion model of rats with hypertension and hyperglycemia
41st Int Stroke Conf (February 17-19, Los Angeles) 2016, Abst TP283
Noguchi, K.; Edo, N.; Ito, Y.; Morishima, Y.; Yamaguchi, K.
Improvement of cerebral blood flow with DS-1040 in a rat thromboembolic stroke model
41st Int Stroke Conf (February 17-19, Los Angeles) 2016, Abst TP271
Lapchak, P.A.; Boitano, P.D.; Noguchi, K.
DS-1040 an inhibitor of the activated thrombin activatable fibrinolysis inhibitor improves behavior in embolized rabbits
41st Int Stroke Conf (February 17-19, Los Angeles) 2016, Abst WP282
A first-in-human, single ascending dose study of DS-1040, an inhibitor of the activated form of thrombinactivatable fibrinolysis inhibitor (TAFIa), in healthy subjects
25th Congr Int Soc Thromb Haemost (ISTH) (June 20-25, Toronto) 2015, Abst PO621-MON
Dow, J.; Puri, A.; McPhillips, P.; Orihashi, Y.; Dishy, V.; Zhou, J.
A drug-drug interaction study of DS-1040 and aspirin in healthy subjects
25th Congr Int Soc Thromb Haemost (ISTH) (June 20-25, Toronto) 2015, Abst PO603-TUE
Noguchi, K.; Edo, N.; Ito, Y.; Yamaguchi, K.
Effect of DS-1040 on endogenous fibrinolysis and impact on bleeding time in rats
25th Congr Int Soc Thromb Haemost (ISTH) (June 20-25, Toronto) 2015, Abst AS145
Noguchi, K.; Edo, N.; Ito, Y.; Maejima, T.; Yamaguchi, K.
DS-1040: A novel selective inhibitor of activated form of thrombin-activatable fibrinolysis inhibitor
25th Congr Int Soc Thromb Haemost (ISTH) (June 20-25, Toronto) 2015, Abst PO203-MON
DS1040b/Aspirin Drug/Drug Interaction Study (NCT02071004)
ClinicalTrials.gov Web Site 2014, February 26
| Patent ID |
Date |
Patent Title |
| US2014178349 |
2014-06-26 |
Cycloalkyl-Substituted Imidazole Derivative |
| US8609710 |
2013-12-17 |
Cycloalkyl-substituted imidazole derivative |
//////DS-1040, DS 1040, phase 2, Daiichi Sankyo Co Ltd, Ischemic stroke
O=C(O)[C@@H](CCCN)Cc1cn(cn1)[C@@H]2CC[C@@H](C)CC2
O=S(=O)(O)c1ccc(C)cc1.O=C(O)[C@@H](CCCN)Cc1cn(cn1)[C@@H]2CC[C@@H](C)CC2





































 67
67 66
66