
RNA, (C-U-U-G-A-C-U-U-U-G-C-U-A-A-G-A-G-C-C-DT-DT), COMPLEX WITH RNA (G-G-C-U-C-U-U-A-G-C-A-A-A-G-U-C-A-A-G-DT-DT)
Duplex of guanylyl-(3′->5′)-guanylyl-(3′->5′)-cytidylyl-(3′->5′)-uridylyl-(3′->5′)-cytidylyl-(3′->5′)-uridylyl-(3′->5′)-uridylyl-(3′->5′)-adenylyl-(3′->5′)-guanylyl-(3′->5′)-cytidylyl-(3′->5′)-adenylyl-(3′->5′)-adenylyl-(3′->5′)-adenylyl-(3′->5′)-guanylyl-(3′->5′)-uridylyl-(3′->5′)-cytidylyl-(3′->5′)-adenylyl-(3′->5′)-adenylyl-(3′->5′)-guanylyl-(3′->5′)-thymidylyl-(3′->5′)-thymidine and thymidylyl-(5′->3′)-thymidylyl-(5′->3′)-cytidylyl-(5′->3′)-cytidylyl-(5′->3′)-guanylyl-(5′->3′)-adenylyl-(5′->3′)-guanylyl-(5′->3′)-adenylyl-(5′->3′)-adenylyl-(5′->3′)-uridylyl-(5′->3′)-cytidylyl-(5′->3′)-guanylyl-(5′->3′)-uridylyl-(5′->3′)-uridylyl-(5′->3′)-uridylyl-(5′->3′)-cytidylyl-(5′->3′)-adenylyl-(5′->3′)-guanylyl-(5′->3′)-uridylyl-(5′->3′)-uridylyl-(5′->3′)-cytidine
Asvasiran sodium (ALN-RSV01),
C401H500N150O290P40,
CAS 1386946-83-3, 870094-26-1
Alnylam Pharmaceuticals
- Originator Alnylam Pharmaceuticals
- Class Antivirals; Small interfering RNA
- Mechanism of Action Nucleocapsid protein modulators; RNA interference
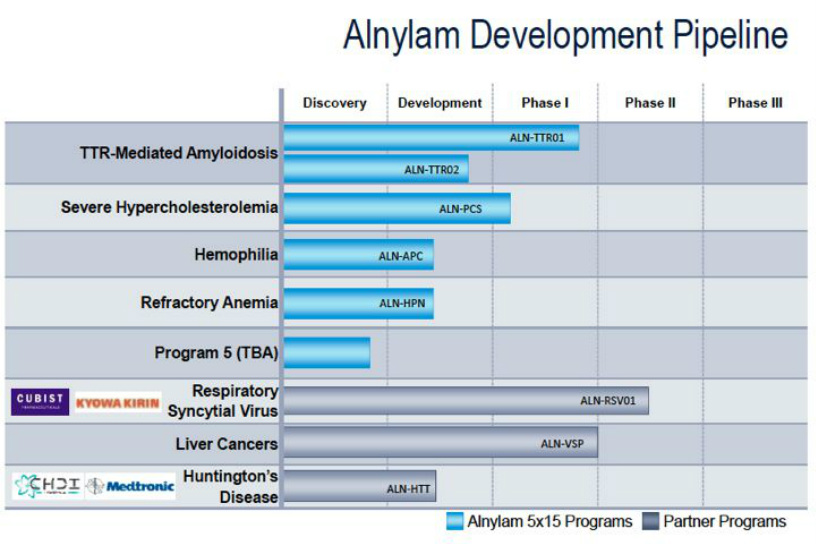
Treatment of Human Respiratory Syncytial Virus (RSV) Infection
Nucleocapsid protein modulators, RNA interference
- 05 Nov 2014 Alnylam receives patent allowance for RNAi technology in USA
- 20 Feb 2014 Suspended – Phase-II for Respiratory syncytial virus infections in USA (Intranasal) (Alnylam Form 10-K filed in February 2014)
- 20 Feb 2014 Suspended – Phase-I for Respiratory syncytial virus infections in Europe (Intranasal) (Alnylam Form 10-K filed in February 2014)
Aerosolised ALN-RSV01 – Alnylam; ALN RSV01; Intranasal ALN-RSV01 – Alnylam
Alnylam, under license from the University of South Alabama, and with Asian licensee Kyowa Hakko Kirin (formerly Kyowa Hakko Kogyo), is developing a nasally administered formulation of asvasiran sodium (ALN-RSV01), an siRNA that targets the respiratory syncytial virus (RSV) N gene and inhibits viral replication, for the potential treatment or prevention of RSV infection.
.In June 2007, a phase II trial was initiated; in January 2008, top-line data were reported . In March 2013, development was ongoing . In August 2008, Kyowa planned to file the drug for marketing approval in 2014. In March 2013, Alnylam was planning on seeking to outlicense the program to continue to advance the program in other regions .
Alnylam is also developing second-generation agents.
Ex-Asian licensee, Cubist Pharmaceuticals, in collaboration with Alnylam, was previously developing the program for the potential treatment or prevention of RSV infection . However, in February 2013, the deal was terminated . Alnylam was also developing an inhaled formulation of asvasiran sodium; however, in February 2014, the drug was no longer listed on the company’s development pipeline.
| WO-2006074346 | |
| WO-2009076679 | |
| WO-2006062596 | |
| WO-2010048590 |
WO 2016022464
WO 2015173701
WO 2015026792
WO 2014209983
WO 2014031784
US 20130273037
Nucleic Acids Research (2012), 40(21), 10585-10595
WO 2011163518
Drugs of the Future (2009), 34(10), 781-783
Current Opinion in Infectious Diseases (2008), 21(6), 639-643
Antiviral Research (2008), 77(3), 225-231



John Maraganore, president and chief executive officer of Alnylam Pharmaceuticals,

Delivering Value with Integrated Communications led by Cynthia Clayton, Vice President, Investor Relations and Corporate Communications at Alnylam Pharmaceuticals

From the left, Alnylam COO Barry Greene, Adrian Dede, Lauren Virnoche, CEO

Dr. Rachel Meyers, Senior Vice President, Research at Alnylam Pharmaceuticals
Dr. Dinah Sah, Vice President of Research and the head of the Alnylam HD team
//////Asvasiran sodium, ALN-RSV01, PHASE 2, Alnylam
SOME OTHER CHEMISTRY
Figure 6: GalNAc–siRNA conjugates.
From Delivery materials for siRNA therapeutics
- Nature Materials12,967–977doi:10.1038/nmat3765
http://www.nature.com/nmat/journal/v12/n11/fig_tab/nmat3765_F6.html
 \
\
http://www.google.com/patents/EP2836595A2?cl=en















Sorry, the comment form is closed at this time.