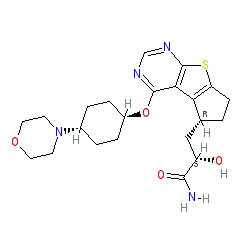
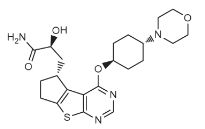
(2S)-2-hydroxy-3-[(3R)-12-{[(1r,4r)-4-(morpholin-4-yl)cyclohexyl]oxy}-7-thia-9,11-diazatricyclo[6.4.0.0²,⁶]dodeca-1(12),2(6),8,10-tetraen-3-yl]propanamide
S)-2-hydroxy-3-((R)-4-(((lr,4R)-4-morpholinocyclohexyl)oxy)-6,7-dihydro-5H-cyclopenta [4,5] thieno [2,3-d] pyrimidin-5-yl)propanamide
- Molecular Weight446.56
ND 2158
IRAK4, 446.2

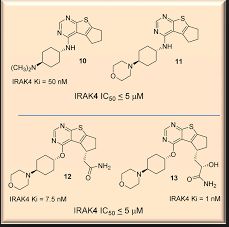
ND-2158 is a potent and selective experimental inhibitor of IRAK4 described in patent WO2013106535 [2] and in a poster presented at the American College of Rheumatology meeting in 2012 (Abstract #1062 in Supplement: Abstracts of the American College of Rheumatology & Association of Rheumatology Health Professionals, Annual Scientific Meeting, November 9-4, 2012 Washington DC, Volume 64, Issue S10, Page S1-S1216).

PATENT
http://www.google.com/patents/WO2013106535A1?cl=en


Scheme II
Example 88: (S)-l-((R)-4-(((lr,4R)-4-morpholinocyclohexyl)oxy)-6,7-dihydro- 5H-cyclopenta[4,5]thieno[2,3-d]pyrimidin-5-yl)butan-2-ol (1-64) and Example 89: (R)-l- ((R)-4-(((lr,4R)-4-morpholinocyclohexyl)oxy)-6,7-dihydro-5H-cyclopenta[4,5]thieno[2,3-
Synthesis of compound 88.1. Note: For the preparation of the starting material compound 29.2, please see Example 29. A solution of
yl)cyclohexyl]oxy]-7-thia-9,l l-diazatricyclo[6.4.0.0[2,6]]dodeca-l(8),2(6),9,l l-tetraen-3- yl]ethan-l-ol (190 mg, 0.47 mmol, 1.00 equiv) in 10 mL of dichloromethane was added Dess- Martin periodinane at 0 °C in a water/ice bath under nitrogen. The resulting mixture was stirred for 2 h at room temperature. After completion of the reaction, the mixture was then diluted with saturated aqueous sodium bicarbonate and extracted with 3 x 30 mL of ethyl acetate. The combined organic layers were dried over sodium sulfate and concentrated under vacuum. The residue was applied onto a silica gel column with ethyl acetate/petroleum ether (1 :5 to 1 : 1) to afford 2-[(3Λ)-12-[[4-^ο 1ιο1ϊη-4-γ1)ογο1ο1ιβχγ1]οχγ]-7-ωΕ-9,11- diazatricyclo[6.4.0.0[2,6]]dodeca-l(8),2(6),9,l l-tetraen-3-yl]acetaldehyde (130 mg, 69%) as a colorless oil. MS (ES): m/z 402 [M+H]+.
Synthesis of Compound 1-64 and Compound 1-65. A solution of [(3i?)-12-[[4- (moφholin-4-yl)cyclohexyl]oxy]-7-thia-9,l l-diazatricyclo[6.4.0.0[2,6]]dodeca-l(8),2(6),9,l l- tetraen-3-yl]acetaldehyde (130 mg, 0.32 mmol, 1.00 equiv) in 5 mL of anhydrous THF was added bromo(ethyl)magnesium (1 M in THF, 0.62 mL, 2.0 equiv) dropwise at 0 °C under nitrogen. The resulting solution was stirred for 4 h at room temperature and then quenched by the addition of saturated aqueous NH4CI and extracted with 3 x 50 mL of DCM/i-PrOH (3:1). The combined organic layers was dried over anhydrous sodium sulfate and concentrated under vacuum. The crude product (150 mg) was purified by preparative HPLC under the following conditions (SHIMADZU): column: SunFire Prep C18, 19*150 mm 5um; mobile phase: water with 0.05% NH4CO3 and CH3CN (6.0% CH3CN up to 54.0% in 25 min); UV detection at 254/220 nm to afford (S)-l-((R)-4-(((lr,4R)-4-moφholinocyclohexyl)oxy)-6,7-dihydro-5H- cyclopenta[4,5]thieno[2,3-d]pyrimidin-5-yl)butan-2-ol (11.8 mg) and (R)-l-((R)-4-(((lr,4R)-4- mo holinocyclohexyl)oxy)-6,7-dihydro-5H-cyclopenta[4,5]thieno[2,3-d]pyrimidin-5-yl)butan- 2-ol (23.9 mg) as white solids.
Example 88 (1-64): MS: 432 (M+H)+. ¾ NMR (300 MHz, CDC13) S 8.47 (s, 2H), 5.24-5.20 (m, 1H), 3.75-3.58 (m, 5H), 3.06-2.93 (m, 2H), 2.70-2.61 (m, 4H), 2.28-1.98 (m, 3H), 1.59-1.41 (m, 10H), 1.28-1.23 (m, 2H),0.95-0.85 (m, 3H).
Example 89 (1-65): MS: 432 (M+H)+. ¾ NMR (300 MHz, CDC13) S 8.47 (s, 2H), 5.25 (m, 1H), 3.71-3.39 (m, 6H), 3.04-2.90 (m, 2H), 2.67-2.55 (m, 5H), 2.34-2.22 (m, 4H), 2.01- 1.81 (m, 3H), 1.64-1.39 (m, 7H), 0.94-0.92 (m, 3H).
WATCH OUT SYNTHESIS COMING…………
PATENT
WO 2014011906
https://www.google.co.in/patents/WO2014011906A2?cl=en
PATENT
WO-2014194242
https://www.google.com/patents/WO2014194242A2?cl=en
Example 49: Synthesis of Intermediate 49.1.
step 1 step 2
35.1 49.1 49.2
step 3 49 3
] Intermediate 49.3 was prepared from 35.1 in a manner analogous to the synthesis of 36.3. Isolated 150 mg of a white solid in 57% overall yield. MS (ES): m/z 402 [M+H]+.
Example 50: Synthesis of Intermediate 50.4.
49.3 50.1 50.2
50.3 50.4
Intermediate 50.4 was prepared from 49.3 in a manner analogous to the synthesis of 1-25, except that HCl/MeOH rather than TBAF/THF was used in the second step. Isolated 124 mg of a white solid in 48% overall yield. MS (ES): m/z 447 [M+H]+. 1H NMR (400 MHz, CDCls): δ 8.46 (s, 1H), 5.28-5.25 (m, 1H), 4.17-4.06 (m, 51H), 3.74-3.72 (m, 5H), 3.37-2.98 (m, 2H), 2.72-2.28 (m, 10H), 2.11-2.08 (m, 2H), 1.79-1.46 (m, 5H).
Example 51: Synthesis of (S)-2-hydroxy-3-((R)-4-(((lr,4R)-4- morpholinocyclohexyl)oxy)-6,7-dihydro-5H-cyclopenta [4,5] thieno [2,3-d] pyrimidin-5- yl)propanamide (1-34) and Example 52: Synthesis of (R)-2-hydroxy-3-((R)-4-(((lr,4R)-4- morpholinocyclohexyl)oxy)-6,7-dihydro-5H-cyclopenta [4,5] thieno [2,3-d] pyrimidin-5- yl)propanamide (1-44)
The racemic 50.4 (1.6 g, 96.5% purity) was separated by Chiral-HPLC with the following conditions (Gilson G x 281): column: Chiralpak AD-H, 2*25 cm Chiral-P(AD-H); mobile phase: phase A: hex (O. P/oDEA) (HPLC grade), phase B: IPA (HPLC grade), gradient: 30% B in 9 min; flow rate: 20 mL/min; UV detection at 220/254 nm. The former fractions (tR = 4.75 min) were collected and evaporated under reduced pressure and lyophilized overnight to afford 1-44 (520 mg) with 100% ee as a white solid. And the latter fractions (tR = 5.82 min) were handled as the former fractions to give the desired 1-34 (510 mg) with 99.6%> ee as a white solid. The ee values of the two isomers were determined by the chiral-HPLC with the following conditions (SHIMADZU-SPD-20A): column: Chiralpak AD-H, 0.46*25 cm, 5um (DAICEL); mobile phase: hex (0.1% TEA): IPA = 85:15; UV detection at 254 nm. Flow rate: 1.0 mL/min. tR (1-44) = 7.939 min and tR (1-34) = 11.918 min.
[00431] Analytical data for 1-44: MS: (ES, m/z) 447 [M+H]+. 1H NMR (400 MHz, CD3OD+CDCI3): δ 8.47 (s, 1H), 5.32-5.22 (m, 1H), 4.08 (dd, 1H), 4.89-4.62 (m, 5H), 3.20-3.10 (m, 1H), 3.05-2.95 (m, 1H), 2.75-2.55 (m, 5H), 2.44-2.38 (m, 2H), 2.34-2.28 (m, 3H), 2.10 (d, 2H), 1.82-1.62 (m, 3H), 1.58-1.40 (m, 2H).
Analytical data for 1-34: MS: (ES, m/z) 447 [M+H]+. 1H NMR (400 MHz, CDC13): δ 8.46 (s, 1H), 5.32-5.22 (m, 1H), 4.15 (t, 1H), 3.73 (t, 4H), 3.59 (td, 1H), 3.19-3.08 (m, 1H), 3.02- 2.92 (m, 1H), 2.78-2.70 (m, 1H), 2.69-2.60 (m, 4H), 2.58-2.20 (m, 5H), 2.10 (d, 2H), 1.75-1.63 (m, 3H), 1.53-1.40 (m, 2H).
Paper
http://pubs.acs.org/doi/abs/10.1021/jm5016044
Recent Advances in the Discovery of Small Molecule Inhibitors of Interleukin-1 Receptor-Associated Kinase 4 (IRAK4) as a Therapeutic Target for Inflammation and Oncology Disorders
Miniperspective

IRAK4, a serine/threonine kinase, plays a key role in both inflammation and oncology diseases. Herein, we summarize the compelling biology surrounding the IRAK4 signaling node in disease, review key structural features of IRAK4 including selectivity challenges, and describe efforts to discover clinically viable IRAK4 inhibitors. Finally, a view of knowledge gained and remaining challenges is provided.
-
78 Romero, D. L.; Robinson, S.; Wessel, M. D.; Greenwood, J. R. IRAK Inhibitors and Uses Thereof. WO201401902, January 16, 2014.
-
Harriman, G. C.; Romero, D. L.; Masse, C. E.; Robinson, S.; Wessel, M. D.; Greenwood, J. R. IRAK Inhibitors and Uses Thereof. WO2014011911A2, January 16, 2014.
-
Harriman, G. C.; Wester, R. T.; Romero, D. L.; Masse, C. E.; Robinson, R.; Greenwood, J. R. IRAK Inhibitors and Uses Thereof. WO2014011906A2, January 16, 2014
| Patent ID | Date | Patent Title |
|---|---|---|
| US2013231328 | 2013-09-05 | IRAK INHIBITORS AND USES THEREOF |
PATENT
WO 2014194242
WO 2013106535
WO 2012097013
| US20070155777 * | Feb 21, 2007 | Jul 5, 2007 | Amgen, Inc. | Antiinflammation agents |
| US20100041676 * | Feb 18, 2010 | Hirst Gavin C | Kinase inhibitors | |
| US20100143341 * | Jun 21, 2006 | Jun 10, 2010 | Develogen Aktiengesellschaft | Thienopyrimidines for pharmaceutical compositions |
| US20120015962 * | Jan 19, 2012 | Nidhi Arora | PYRAZOLO[1,5a]PYRIMIDINE DERIVATIVES AS IRAK4 MODULATORS | |
| US20120283238 * | Nov 8, 2012 | Nimbus Iris, Inc. | Irak inhibitors and uses thereof |
| References |
| 1. Chaudhary D, Robinson S, Romero DL. (2015) Recent Advances in the Discovery of Small Molecule Inhibitors of Interleukin-1 Receptor-Associated Kinase 4 (IRAK4) as a Therapeutic Target for Inflammation and Oncology Disorders. J. Med. Chem., 58 (1): 96-110. [PMID:25479567] |
| 2. Harriman GC, Wester RT, Romero DL, Robinson S, Shelley M, Wessel MD, Greenwood JR, Masse CE, Kapeller-Libermann R. (2013) Irak inhibitors and uses thereof. Patent number: WO2013106535. Assignee: Nimbus Iris, Inc.. Priority date: 18/07/2013. Publication date: 10/01/2012. |
http://nimbustx.com/sites/default/files/uploads/posters/irak4_nimbus_acr_poster_2012_small.pdf
///////ND 2158, IRAK4, ND-2158, NIMBUS, 1388896-07-8
NC(=O)C(CC1CCc2c1c1c(ncnc1s2)OC1CCC(CC1)N1CCOCC1)O
C1CC(CCC1N2CCOCC2)OC3=C4C5=C(CCC5CC(C(=O)N)O)SC4=NC=N3
















Sorry, the comment form is closed at this time.