Bassas, O.; Huuskonen, J.; Rissanen, K.; Koskinen, A.M.P. ’A Simple Organocatalytic Enantioselective Synthesis of Pregabalin.’ Eur. J. Org. Chem. 2009, 1340-1351.
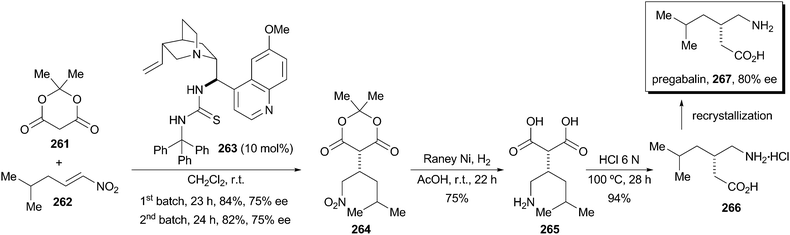
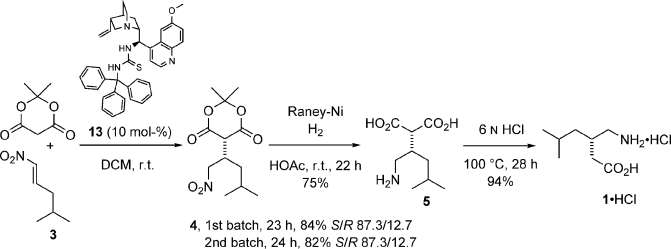
This paper describes a new procedure for the enantioselective synthesis of the important anticonvulsant drug Pregabalin, which shows biological properties as the (S) enantiomer only. The key step of the synthetic sequence is the Michael addition reaction of Meldrum’s acid to a nitroalkene mediated by a quinidine derived thiourea. A variety of novel catalysts bearing different groups at the thiourea moiety were synthesized and tested. The most successful catalyst that incorporates a trityl substituent provided up to 75 % ee of (S)-4. The conjugate addition reaction was carried out on a multigram scale with low loadings of catalyst (10 mol-%). Moreover, the catalyst can be recycled showing the same capability in chemical yield and asymmetric induction. Then, hydrogenation of nitroalkane 4 followed by decarboxylation of diacid 5 provides Pregabalin hydrochloride in 59 % overall yield. Enantioenrichment by crystallization of the free amino acid 1 improves the (S)/(R) enantiomeric ratio to 9:1.
Author Information
- †X-ray crystallography.
Email: Ari M. P. Koskinen (ari.koskinen@hut.fi)
http://onlinelibrary.wiley.com/doi/10.1002/ejoc.200801220/abstract
Jyväskylä, Finland
TAKE A TOUR
Jyväskylä, Finland
-
Jyväskylä – Wikipedia, the free encyclopedia
en.wikipedia.org/wiki/JyväskyläJyväskylä (Finnish pronunciation: [ˈjyvæsˌkylæ]) is a city and municipality in CentralFinland in the western part of the Finnish Lakeland. It is the largest city in
…





///////////













Sorry, the comment form is closed at this time.