Apr 102015


Venlafaxine
CAS : 93413-69-5
CAS Name: 1-[2-(Dimethylamino)-1-(4-methoxyphenyl)ethyl]cyclohexanol
Additional Names: (±)-1-[a-[(dimethylamino)methyl]-p-methoxybenzyl]cyclohexanol; N,N-dimethyl-2-(1-hydroxycyclohexyl)-2-(4-methoxyphenyl)ethylamine; venlafexine
Molecular Formula: C17H27NO2
Molecular Weight: 277.40
Percent Composition: C 73.61%, H 9.81%, N 5.05%, O 11.54%
 SEE
SEEpart 1………http://orgspectroscopyint.blogspot.in/2015/04/venlafaxine.html / http://newdrugapprovals.org/2015/04/09/venlafaxine-part-12/
part 2……..http://newdrugapprovals.org/2015/04/09/venlafaxine-22/
PART 3…..http://orgspectroscopyint.blogspot.in/2015/04/venlafaxine-part-33.html
SEE ALSO
http://www.google.com/patents/WO2008059525A2?cl=en
WILL BE UPDATED………..
Venlafaxine (brand names: Effexor, Effexor XR and Trevilor) is an antidepressant of the serotonin-norepinephrine reuptake inhibitor (SNRI) class.[3][4][5] This means it increases the concentrations of the neurotransmitters serotonin and norepinephrine in the body and the brain. First introduced by Wyeth in 1993, now marketed by Pfizer, it is licensed for the treatment of major depressive disorder (MDD), generalised anxiety disorder (GAD), panic disorder and social phobia.[6][7]
Comparative efficacy and acceptability of 12 new-generation antidepressants: a multiple-treatments meta-analysis have shown venlafaxine, alongside mirtazapine, escitalopram and sertraline were significantly more efficacious.[8] Remission rates (defined as a HAM-D score of 7 or less) were 58% for venlafaxine plus mirtazapine.[9]
The rate of life-threatening or lethal outcomes for suicidal overdoses of venlafaxine is lower than for the TCAs, MAOIs and bupropionand comparable to several of the SSRIs.[10] It is metabolised in the body into another antidepressant drug called desvenlafaxine (O-desmethylvenlafaxine) which is also sold as an antidepressant, under the brand name Pristiq.[11]
Both venlafaxine and paroxetine have been linked to the most severe discontinuation symptomes.
In 2007, venlafaxine was the sixth most commonly prescribed antidepressant on the U.S. retail market, with 17.2 million prescriptions.[12]
Chemistry
The chemical structure of venlafaxine is designated (R/S)-1-[2-(dimethylamino)-1-(4 methoxyphenyl)ethyl] cyclohexanol hydrochloride or (±)-1-[a [a- (dimethylamino)methyl] p-methoxybenzyl] cyclohexanol hydrochloride, and it has the empirical formula of C17H27NO2. It is a white to off-white crystalline solid. Venlafaxine is structurally and pharmacologically related to the atypical opioid analgesictramadol, and more distantly to the newly released opioid tapentadol, but not to any of the conventional antidepressant drugs, including tricyclic antidepressants, SSRIs, MAOIs, or RIMAs.[66]

 |
|
 |
|
| Systematic (IUPAC) name | |
|---|---|
| (RS)-1-[2-dimethylamino-1-(4-methoxyphenyl)-ethyl]cyclohexanol | |
| Clinical data | |
| Trade names | Effexor XR, Effexor, Trevilor |
| AHFS/Drugs.com | monograph |
| Licence data | US Daily Med:link |
|
|
|
|
| Oral | |
| Pharmacokinetic data | |
| Bioavailability | 42±15%[1] |
| Protein binding | 27±2% (parent compound), 30±12% (active metabolite,desvenlafaxine)[2] |
| Metabolism | Hepatic (~50% of the parent compound is metabolised on first pass through the liver)[1][2] |
| Half-life | 5±2 h (parent compound for immediate release preparations), 15±6 h (parent compound for extended release preparations), 11±2 h (active metabolite)[1][2] |
| Excretion | Renal (87%; 5% as unchanged drug; 29% asdesvenlafaxine and 53% as other metabolites)[1][2] |
| Identifiers | |
| 93413-69-5 |
|
| N06AX16 | |
| PubChem | CID 5656 |
| DrugBank | DB00285 |
| ChemSpider | 5454 |
| UNII | GRZ5RCB1QG |
| ChEBI | CHEBI:9943 |
| ChEMBL | CHEMBL637 |
| Chemical data | |
| Formula | C17H27NO2 |
| 277.402 g/mol | |

Derivative Type: Hydrochloride
CAS : 99300-78-4
Manufacturers’ Codes: Wy-45030
Trademarks: Effexor (Wyeth)
Molecular Formula: C17H27NO2.HCl
Molecular Weight: 313.86
Percent Composition: C 65.06%, H 8.99%, N 4.46%, O 10.20%, Cl 11.30%
Properties: White to off-white crystalline solid from methanol/ethyl acetate, mp 215-217°. Soly (mg/ml): 572 water. Partition coefficient (octanol/water): 0.43.
Melting point: mp 215-217°
Log P: Partition coefficient (octanol/water): 0.43
Derivative Type: (+)-Form
Properties: Crystals from ethyl acetate, mp 102-104°. [a]D25 +27.6° (c = 1.07 in 95% ethanol).
Melting point: mp 102-104°
Optical Rotation: [a]D25 +27.6° (c = 1.07 in 95% ethanol)
Derivative Type: (+)-Form hydrochloride
Manufacturers’ Codes: Wy-45655
Properties: Crystals from methanol/ether, mp 240-240.5°. [a]D25 -4.7° (c = 0.945 in ethanol).
Melting point: mp 240-240.5°
Optical Rotation: [a]D25 -4.7° (c = 0.945 in ethanol)
Derivative Type: (-)-Form
Properties: Crystals from ethyl acetate, mp 102-104°. [a]D25 -27.1° (c = 1.04 in 95% ethanol).
Melting point: mp 102-104°
Optical Rotation: [a]D25 -27.1° (c = 1.04 in 95% ethanol)
Derivative Type: (-)-Form hydrochloride
Manufacturers’ Codes: Wy-45651
Properties: Crystals from methanol/ether, mp 240-240.5°. [a]D25 +4.6° (c = 1.0 in ethanol).
Melting point: mp 240-240.5°
Optical Rotation: [a]D25 +4.6° (c = 1.0 in ethanol)
Therap-Cat: Antidepressant.
Keywords: Antidepressant; Serotonin Noradrenaline Reuptake Inhibitor (SNRI).
1H NMR

HSQC

1H NMR PREDICT OF HCL

13C NMR PREDICT OF HCL

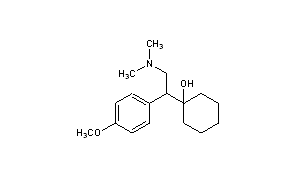
BASE


Literature References:
Serotonin noradrenaline reuptake inhibitor (SNRI). Prepn: G. E. M. Husbands et al., EP 112669; US4535186 (1984, 1985 both to Am. Home Prods.);
and resolution of isomers: J. P. Yardley et al., J. Med. Chem. 33, 2899 (1990). Receptor binding studies: E. A. Muth et al., Biochem. Pharmacol. 35, 4493 (1986).
HPLC determn in biological fluids: D. R. Hickset al., Ther. Drug Monit. 16, 100 (1994).
Clinical pharmacokinetics: K. J. Klamerus et al., J. Clin. Pharmacol. 32, 716 (1992).
Clinical trial in major depression: E. Schweizer et al., J. Clin. Psychopharmacol. 11, 233 (1991).
Review of pharmacology and clinical efficacy in depression: S. A. Montgomery, J. Clin. Psychiatry 54, 119-126 (1993).
Clinical trial in generalized anxiety disorder: A. J. Gelenberg et al., J. Am. Med. Assoc. 283, 3082 (2000).
External links[Drug information
- U.S. Food and Drug Administration information on Effexor
- Efexor patient information leaflet (UK)
- Effexor XR prescribing information for healthcare professionals (pdf) (USA only) Archived from the original on 17 September 2006.
- Detailed Patient/Parent Information on Effexor
- List of international brand names for Venlafaxine
- U.S. National Library of Medicine: Drug Information Portal -Venlafaxine
Diagnostic tools
Patient experiences
P.S.
: The views expressed are my personal and in no-way suggest the views
of the professional body or the company that I represent.
: The views expressed are my personal and in no-way suggest the views
of the professional body or the company that I represent.
P.S.
: The views expressed are my personal and in no-way suggest the views
of the professional body or the company that I represent.
: The views expressed are my personal and in no-way suggest the views
of the professional body or the company that I represent.
P.S.
: The views expressed are my personal and in no-way suggest the views
of the professional body or the company that I represent.
: The views expressed are my personal and in no-way suggest the views
of the professional body or the company that I represent.

 COCK WILL TEACH YOU NMR
COCK WILL TEACH YOU NMR COCK SAYS MOM CAN TEACH YOU NMR
COCK SAYS MOM CAN TEACH YOU NMR
 DRUG APPROVALS BY DR ANTHONY MELVIN CRASTO …..FOR BLOG HOME CLICK HERE
DRUG APPROVALS BY DR ANTHONY MELVIN CRASTO …..FOR BLOG HOME CLICK HERE













Sorry, the comment form is closed at this time.