
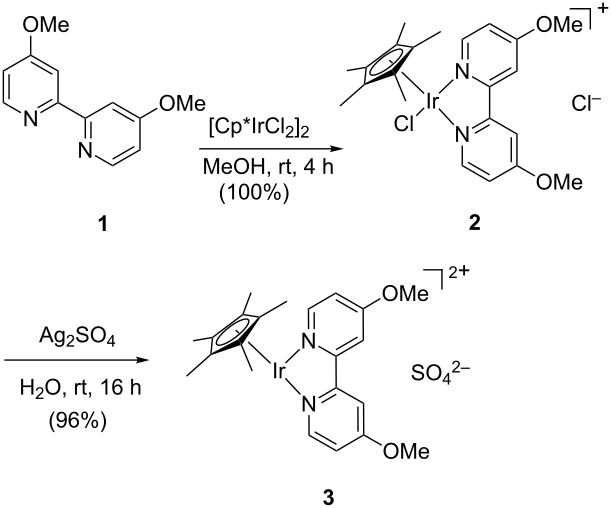
http://pubs.rsc.org/en/Content/ArticleLanding/2015/OB/C4OB02376E#!divAbstract
A monolith immobilised iridium Cp* catalyst for hydrogen transfer reactions under flow conditions
DOI: 10.1039/C4OB02376E

Dr. David Balcells, Prof. Odile Eisenstein, Prof. Robert H Crabtree, Agusti Lledos Departament de Quimica, Universitat Autonoma de Barcelona, Bellaterra, Spain; Institut Charles Gerhardt, Universite Montpellier 2, Montpellier, France; Department of Chemistry, Yale University, New Haven, United States
The development of a new energy model is a major challenge in modern chemistry. The climate change and the raise of oil prices prompt the development of clean and cheap energy resources. In this field, artificial photosynthesis is one of the most promising solutions.1 The catalytic oxidation of water to dioxygen is a fundamental part of this process. The mononuclear iridium complex Cp*Ir(ppy)(Cl) (ppy = phenylpyridine) is one of the most efficient catalysts reported for this reaction (Figure).2 DFT calculations support the oxo complex Cp*IrO(ppy) as the active species. The electronic structure of this complex is characterized by having the antibonding p*(Ir=O) orbitals half-occupied. The calculations suggest that the reaction mechanism consists of an intermolecular attack of water to the oxo ligand. This reaction involves the formation of the O-O bond and a proton transfer, which is assisted by the molecules of water solvating the catalyst.

Figure. Iridium-catalyzed water oxidation.
References
(1) Hammarström, L.; Hammes-Schiffer, S. Acc. Chem. Res. 2009, 42, 1859-1860.
(2) Hull, J. F.; Balcells, D.; Blakemore, J. D.; Incarvito, C. D.; Eisenstein, O.; Brudvig, G. W.; Crabtree, R. H. J. Am. Chem. Soc.2009, 131, 8730-8731.

The N-Alkylation of Sulfonamides with Alcohols in Water Catalyzed by the Water-Soluble Iridium Complex {Cp*Ir[6,6′-(OH)2bpy](H2O)}[OTf]2
Article first published online: 13 JAN 2014
DOI: 10.1002/adsc.201300711
http://onlinelibrary.wiley.com/doi/10.1002/adsc.201300711/abstract
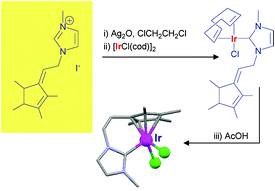
http://www.beilstein-journals.org/bjoc/single/articleFullText.htm?publicId=1860-5397-9-110













Sorry, the comment form is closed at this time.