
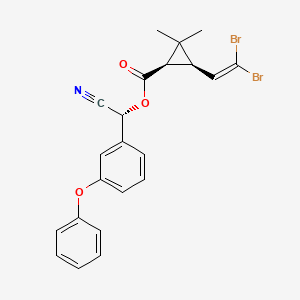
Deltamethrin
Deltamethrin is a pyrethroid ester insecticide.
: AC1O570W; NRDC-161; 52918-63-5; FMC-45498; ZINC01532093; RU 23938; UNII-26515THS0L component OWZREIFADZCYQD-BJLQDIEVSA-N
Purified deltamethrin is an off-white powder that has none to slight musty odour. Technical deltamethrin (purity 98.9-99.3%w/w) melts at 98.1-99.4 °C and decomposes before boiling (245-320 °C). The density is 1.5 g/cm3
at 20°C and the solubility in water is < 5µg/l at pH 6.2and 20 °C (based on the LOQ of the HPLC-method used for quantification). Deltamethrin is notconsidered to be able to dissociate within the environmentally relevant pH range due to the lack
of functional groups with acidic or alkaline properties. The vapour pressure is 1.24 x 10-8 Pa at
25 °C and the Henry’s law constant of 1.252 x 10-3 Pa.m3.mol-1 indicates that volatilisation is
not expected to significantly contribute to the dissipation of deltamethrin in the environment.
The Log Pow is 4.6 in distilled water (pH not stated) which indicates that deltamethrin may bioaccumulate. The following solvent solubilities were determined for deltamethrin (g/l): 300-600 (acetone), 60-75 (acetonitrile), >600 (1,2-dichloroethane), 200-300 (DMSO and ethyl actetate), 2.47 (n-heptane), 8.15 (methanol) and 150-200 (p-xylene). Deltamethrin is not highly
flammable, auto-flammable, exploosive or oxidizing and does not react with the packaging material (black plastic pouches).
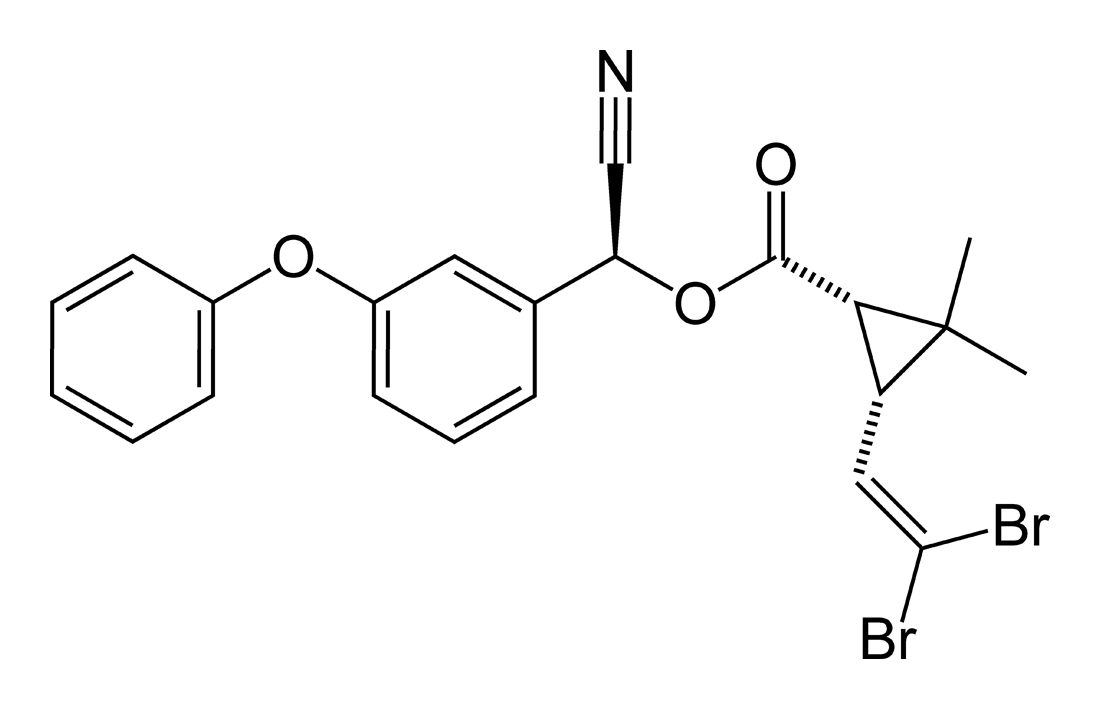
Molecular formula: C22H19Br2NO3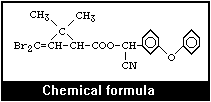
Deltamethrin is the first pyrethroid composed of a single isomer of 8 stereoisomers selectively prepared by the esterification of [1R, 3R or cis]-2,2-dimethyl-3-(2,2-dibromovinyl) cyclopropanecarboxylic acid with (alphaS)- or (+)-alpha-cyano-3- phenoxybenzyl alcohol or by selective recrystallization of the racemic esters obtained by esterification of the (1R, 3R or cis)- acid with the racemic or [alphaR, alphaS, or alphaRS or ±]-alcohol (Elliott et al., 1974). Thus, its stereospecific structure (4) is the ester of [1R, 3R or cis]-acid with (alphaS)-alcohol. The acid is a characteristic dibromo analogue of chrysanthemic acid.
Deltamethrin (S-α-cyano-3-phenoxybenzyl-(1R,3R)-3-(2,2-dibromovinyl)-2,2-dimethylcyclopropanecarboxylic acid ester) is an insecticide from the class of the pyrethroids and has been used extensively and for a long time for controlling pests (C. D. S. Tomlin, The Pesticide Manual, 11th Edition, British Crop Protection Council, Farnham 1997).
Deltamethrin is a pyrethroid insecticide that kills insects on contact and through digestion. It is used to control apple and pear suckers, plum fruit moth, caterpillars on brassicas, pea moth, aphids (apples, plums, hops), winter moth (apples and plums), codling and tortrix moths (apples). Control of aphids, mealy bugs, scale insects, and whitefly on glasshouse cucumbers, tomatoes, peppers, potted plants, and ornamentals. It also controls numerous insect pests of field crops. Formulations include emulsifiable concentrates, wettable powders, ULV and flowable formulations and granules. There are no known incompatibilities with other common insecticides and fungicides
The pyrethroids with which the present invention is concerned are crystallizable esters having at least one asymmetric carbon atom to which an epimerizable proton is attached. The more pesticidally active pyrethroids additionally contain at least one and usually two or more other asymmetric carbon atoms and therefore comprise isomeric mixtures wherein one or more of the isomers are more pesticidally active than the others. Representative of such pyrethroids are the alpha-cyanobenzyl esters of the formula (A) :
wherein R1 is halogen, haloalkyl, alkenyl or halo- alkenyl; each R2 independently is halogen, alkyl, halo- alkyl, alkoxy, phenyl, phenoxy, phenylalkyl, substituted phenyl and substituted phenylalkyl wherein the substituents include one or more of alkyl, halogen, haloalkyl, nitro, hydroxy and cyano; and n is 0-5, preferably 1-3. In the above formula the asymmetric carbon atoms are marked 1, 3 and alpha. All of the substituents on a host group may be the same, or the substituents may be different. Alkyl and alkoxy may contain 1-8 carbon atoms, preferably 1-4 carbon atoms. Alkenyl may comprise 2-8 carbon atoms, preferably 2-4 carbon atoms. Halogen includes fluorine, chlorine and bromine. A typical phenylalkyl group is benzyl. Substituted phenyl includes tolyl, xylyl, trichlorophenyl and trifluoro- methylphenyl. Substituted phenylalkyl includes methyl- benzyl, trichlorobenzyl and trifluoromethylbenzyl.
The foregoing and other pyrethroids are well known as disclosed, for example, in Kirk-Othmer, Encyclopedia of Chemical Technology, Second Edition, Vol. 13, pages 456-458, in the following U.S. Patents: 4,024,163 – Elliot et al (NRDC)
4,133,826 – Warnant et al (Roussel Uclaf)
4,136,195 – Warnant et al (Roussel Uclaf)
4,213,916 – Davies et al (Shell)
4,287,208 – Fuchs et al (Bayer) 4,308,279 – Smeltz (FMC)
4,427,598 – Mason et al (Shell)
4,512,931 – Robson (ICI)
4,544,508 – Fuchs et al (Bayer)
4,544,510 – Van Berkel et al (Shell) 4,560,515 – Stoutamire et al (Shell)
4,582,646 – Stoutamire et al (Shell)
4,670,464 – Doyle et al (ICI)
4,681,969 – Williams et al (ICI) and in the following PCT patent publications:
WO 86/04215 – Hidasi et al (Chinoin)
WO 86/04216 – Hidasi et al (Chinoin)
Preferred pyrethroids convertible to more active isomers in accordance with the present invention are those of formula A wherein R1 is dihalovinyl or tetrahalopropenyl, R2 is phenoxy, and n is l. The more preferred pyrethroids are those wherein n is 1, R1 is dihalovinyl or tetrahalopropenyl and R2 is phenoxy; and those wherein n is 2, R1 is dihalovinyl or tetrahalopropenyl and one R2 is fluorine and the other R2 is phenoxy. The latter preferred compounds are isomeric mixtures having the common name “cyfluthrin” when R1 is dichlorovinyl, n is 2 and one R2 is fluorine. When R1 is dichlorovinyl, n is 1 and R2 is phenoxy, the mixtures have the common name “cypermethrin.”
Cypermethrin contains four cis and four trans isomers designated I-VIII as follows:
cis isomers I. (S) (α-cyano)(3-phenoxyphenyl)methyl 1R, cis-3-
(2,2-dichloroethenyl)-2,2-dimethylcyclopropane- carboxylate (abbreviated 1R, cis S)
II. (R)(α-cyano)(3-phenoxyphenyl)methyl 1S, cis-3- (2,2-dichloroethyl)-2,2-dimethylcyclopropane- carboxylate (abbreviated 1S, cis R)
III. (S)(α-cyano)(3-phenoxyphenyl)methyl 1S, cis-3- (2,2-dichloroethenyl)-2,2-dimethylcyclopropane- carboxylate (abbreviated 1S, cis S)
IV. (R)(α-cyano)(3-phenoxyphenyl)methyl 1R, cis-3-
(2,2-dichloroethenyl)-2,2-dimethylcyclopropane- carboxylate (abbreviated 1R, cis R)
trans isomers V. The trans isomer of I (abbreviated 1R, trans S)
VI. The trans isomer of II (abbreviated 1S,trans R)
VII. The trans isomer of III (abbreviated 1S,trans S)
VIII. The trans isomer of IV (abbreviated 1R, trans R)
Cyfluthrin and other pyrethroids to which the invention is applicable comprise similar isomericmixtures.
It is known that the most insecticidally active isomers of the foregoing eight isomers are I and V, and that enantiomer pairs I/II (abbreviated cis-2) and V/VI (abbreviated trans-2) are more insecticidally active than the enantiomer pairs III/IV (abbreviated cis-1) and VII/VIII (abbreviated trans-1). It is extremely difficult and commercially impractical to separate the more active isomers such as I and V from the complex isomer mixtures produced in the usual pyrethroid synthesis. Accordingly, efforts to produce more pesticidally active pyrethroids have focused on techniques for converting less active isomers in the synthesis product mixtures to more active isomers, i.e., to enrich isomeric mixtures with respect to the more active isomers, thus avoiding complex resolution procedures and the loss represented by discard of less active isomers.
Nevertheless, even when the isomeric mixtures have been converted rather than resolved, the conversion procedures have not been commercially practical because of poor yields, usually due to production of undesired by-product, often comprising as many isomers as the desired product, and because of time-consuming multiple steps, high temperatures and/or the need to recover expensive reagents. In the case of cypermethrin the major by-product is (R,S)-2-oxo-1,2-bis(3-phenoxyphenyl) ethyl cis- and trans-3-(2,2-dichloroethenyl)-2,2- dimethylcyclopropanecarboxylate, an eight isomer mixture commonly called the “benzoin by-product.” Similar byproducts are encountered in the synthesis of other pyrethroids such as cyfluthrin. Representative of prior efforts to convert isomer mixtures to more active species are the procedures disclosed in U.S. Patents 4,213,916, 4,308,279, 4,544,510, 4,544,508, 4,512,931, 4,427,598, 4,670,646 and 4,681,969 and the two PCT patent publications cited above.

Usage
Deltamethrin products are among the most popular and widely used insecticides in the world[citation needed] and have become very popular with pest control operators and individuals in the United States.[1] This material is a member of one of the safest classes of pesticides: synthetic pyrethroids. This pesticide is highly toxic to aquatic life, particularly fish, and therefore must be used with extreme caution around water. Although generally considered safe to use around humans, it is still neurotoxic to humans. Deltamethrin is able to pass from a woman’s skin through her blood and into her breast milk.[2]
There are many uses for deltamethrin, ranging from agricultural uses to home pest control. Deltamethrin has been instrumental in preventing the spread of diseases carried by tick-infested prairie dogs, rodents and other burrowing animals[citation needed]. It is helpful in eliminating and preventing a wide variety of household pests, especially spiders, fleas, ticks, carpenter ants, carpenter bees,cockroaches and bed bugs. Deltamethrin is also one of the primary ingredients in ant chalk.
Production
Deltamethrin is a pyrethroid composed of a single isomer of 8 stereoisomers selectively prepared by the esterification of (1R,3R)- orcis-2,2-dimethyl-3-(2,2-dibromovinyl)cyclopropanecarboxylic acid with (alpha,S)- or (+)-alpha-cyano-3-phenoxybenzyl alcohol or by selective recrystallization of the racemic esters obtained by esterification of the (1R,3R)- or cis-acid with the racemic or (alpha–R,alpha–S, or alpha–R/S)- or + or − alcohol.
…………………………….
Evolution of an industrial process: deltamethrin synthesis
Chemistry&Industry (London, United Kingdom) (1984), (6), 199-204
……………………….
http://www.sciencedirect.com/science/article/pii/S0040402001801307
Very efficient enantioselective syntheses of (1R)-![]() -and
-and ![]() -hemicaronaldehydes precursors of (1R)-trans chrysanthemic acid and its (1R)-cis dibromovinyl analogue starting from natural tartaric acid or D-mannitol are described. They are based on the reaction between isopropylidenetriphenylphosphorane or isopropylidenediphenylsulfurane and chiral γ-alkoxy-α,β-unsaturated esters. The general problem of the diastereoselective addition to such esters is discussed.
-hemicaronaldehydes precursors of (1R)-trans chrysanthemic acid and its (1R)-cis dibromovinyl analogue starting from natural tartaric acid or D-mannitol are described. They are based on the reaction between isopropylidenetriphenylphosphorane or isopropylidenediphenylsulfurane and chiral γ-alkoxy-α,β-unsaturated esters. The general problem of the diastereoselective addition to such esters is discussed.
………………………….
http://www.sciencedirect.com/science/article/pii/0040403988853413
Isopropylidenediphenylsulfurane reacts with γ-alkoxy α,β-unsaturated esters and produces γ-alkoxy cyclopropane carboxylates. This reaction is almost stereospecific and takes place with very high asymmetric induction. The stereochemistry of the resulting cyclopropane derivative proved independent of the stereochemistry of the carbon-carbon double bond of the starting ester. This reaction has been successfully applied to the enantioselective synthesis of deltamethrin (the most biologically active insecticide) from natural tartaric acid.
(+)-4 α-Acetyl-2-carene (6), readily available from (+)-3-carene (5), was converted to (1R)-![]() -(+)-3-(2′,2′-dihalovinyl)-2, 2-dimethyl-cyclopropane-1-carboxylic acids (21) and (22) in eleven steps and in overall yields of 23% and 14%, respectively. Alternatively, (-)-5-keto-3-carene (23), an oxidation product of (+)-3-carene (5) was converted to (1R)-
-(+)-3-(2′,2′-dihalovinyl)-2, 2-dimethyl-cyclopropane-1-carboxylic acids (21) and (22) in eleven steps and in overall yields of 23% and 14%, respectively. Alternatively, (-)-5-keto-3-carene (23), an oxidation product of (+)-3-carene (5) was converted to (1R)-![]() -(-)-permethrin (1) in five steps and in an overall yield of 20%. in yet another flexible approach, (-)-(23) was converted to (1R)-
-(-)-permethrin (1) in five steps and in an overall yield of 20%. in yet another flexible approach, (-)-(23) was converted to (1R)-![]() -(+)-(21) and (1R)-
-(+)-(21) and (1R)-![]() -(+)-(22) in seven steps and in an overall yields of 33% and 23%, respectively. These results coupled with the literature reports for the conversion of (1R)-
-(+)-(22) in seven steps and in an overall yields of 33% and 23%, respectively. These results coupled with the literature reports for the conversion of (1R)-![]() -(+)-(21) and (22) to (1R)-
-(+)-(21) and (22) to (1R)-![]() -(-)-(1), (1R)-cis-(+)-cypermethrin (2) and (1R)-
-(-)-(1), (1R)-cis-(+)-cypermethrin (2) and (1R)-![]() -(+)-deltamethrin (decis) (3) constitute two efficient methods for the synthesis of (1R)-
-(+)-deltamethrin (decis) (3) constitute two efficient methods for the synthesis of (1R)-![]() -synthetic pyrethroid from (+)-3-carene (5).
-synthetic pyrethroid from (+)-3-carene (5).
………………………..
1R-cis-2,2-Dimethyl-3-(2,2-dibromovinyl)cyclopropane carboxylic acid1 (1), the acid moiety of the highly potent photostable pyrethroid deltamethrin (2) has been obtained either by a Wittig reaction on 1R-cis-caronaldehyde ester (3) employing 1, 1-dibromomethylenetriphenylphosphorane or from the bicyclic tribromo-lactone2,3 (4) by reaction with zinc and acetic acid. Lactone (4) is thus an important intermediate in the deltamethrin synthesis.
http://www.tandfonline.com/doi/abs/10.1080/00397918908050947#.VESwr2eSyvM
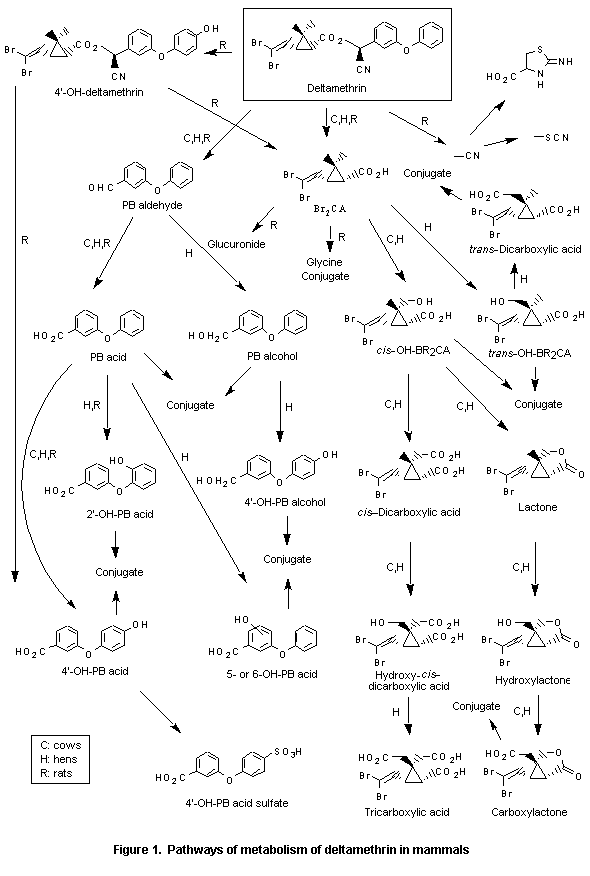
Physical and Chemical Properties Technical grade deltamethrin contains more than 98% deltamethrin (FAO/WHO, 1981). It is stable to heat (6 months at 40 °C), light, and air, but unstable in alkaline media (FAO/WHO, 1981; Meister et al., 1983; Worthing & Walker, 1983). Some physical and chemical properties are listed in Table 1, and the chemical composition of various stereoisomeric mixtures is shown in Table 2. Table 1. Some physical and chemical properties of deltamethrin ------------------------------------------------------------------- Physical state crystalline powder Colour colourless Odour odourless Density (20 °C) 0.5 g/cm3 Relative molecular mass 505.24 Melting point (°C) 98-101 Boiling point decomposes above 300 °C Water solubility (20 °C) < 0.002 mg/litre (practically insoluble) Solubility in organic solublea solvents Vapour pressure (25 °C) 2.0 x 10-6 Pa n-Octanol-water 5.43 partition coefficient (Log Pow) ------------------------------------------------------------------- a Acetone (500 g/litre), ethanol (15 g/litre), cyclohexanone (750 g/litre), dioxane (900 g/litre), xylene (250 g/litre), ethyl acetate. 2.3 Analytical Methods Methods for the determination of deltamethrin residues and the analysis of environmental samples, and products are summarized in Table 3. To analyse technical grade deltamethrin, a mixture of deltamethrin and diphenylamine (an internal standard) was injected in a high-performance liquid chromatograph equipped with a UV- detector (Mourot et al., 1979). The Joint FAO/WHO Codex Alimentarius Commission has published recommendations for methods for the determination of deltamethrin residues (FAO/WHO 1985b). A further review of analytical methods for deltamethrin has been made by Vaysse et al. (1984). Table 2. Chemical identity of deltamethrins of various stereoisomeric compositions --------------------------------------------------------------------------------------------------------------- Common name CA Index name (9CI) Stereoisomeric Synonyms and trade names CAS Registry No. compositionc NIOSH Accession No.a Stereospecific nameb --------------------------------------------------------------------------------------------------------------- Deltamethrin Cyclopropanecarboxylic acid, (4) Decamethrin, Decis, 52918-63-5 3-(2,2-dibromovinyl)-2,2-dimethyl-, K-Othrine, NRDC 161, GZ1233000a alpha-cyano(3-phenoxyphenyl)methyl ester, WHO 1998, K-Obiol, Butox [1R-[1 (S*), 3 R]]-, Butoflin, Cislin, FMC 45498 RU 22974 (S)-alpha-cyano-3-phenoxybenzyl (1R, cis)-2,2-dimethyl-3-(2,2-di- bromovinyl)cyclopropanecarboxylate d- cis-Deltamethrin same as deltamethrin - Decamethrin, Decis 52820-00-5 GZ1240000a (S)-alpha-cyano-3-phenoxybenzyl (d, cis)-2,2-dimethyl-3-(2,2-di- bromovinyl)cyclopropanecarboxylate --------------------------------------------------------------------------------------------------------------- a Registry of Toxic Effects of Chemical Substances (RTECS) (1981-82 edition). b (1R), d, (+) or (1S), 1, (-) in the acid part of deltamethrin signifies the same stereospecific conformation, respectively. c The number in the parenthesis identifies the structure shown in the figures of stereoisomers. Table 3. Analytical methods for deltamethrin --------------------------------------------------------------------------------------------------------------------------------- Sample Extraction Sample preparation Determination: MDCb % Recovery Reference solvent ----------------------------------- (mg/kg) (fortification Partition Clean up GLC or HPLC level in column elution condition; detector, mg/kg) carrier flow, column, temp, R.T.a --------------------------------------------------------------------------------------------------------------------------------- RESIDUE ANALYSIS apple n-hexane/ ext.sol.c/ silica gel CH2Cl2 ECD-GC; N2; 0.01 105(0.1), 100(1.0) 1 acetone H2O 50 ml/min; 1 m (1/1) 3% OV-7; 235 °C pear 0.01 125(0.1), 98(1.0) cabbage 0.01 130(0.1), 118(1.0) potato 0.01 126(0.1), 97(1.0) apple, acetonitrile petroleum Florisil ether/ EDC-GC; 1.2 m 0.005 85-100(0.02-0.1) 2 peach, ether/H2O n-hexane DC-200, OV-1 or grape, (1/4) OV-101; 245 °C, tomato 10-12 min wheat methanol n-hexane alumina HPLC; 235 nm; 87(2.0) 3 grain 30 cm; uBondapak; C 18; methanol/H2O (4/1); 2.5 ml/min wheat n-hexane Florisil ether/ ECD-GC; N2; 91 4 petroleum 75 ml/min; 0.6 m ether (1/9) 5% SE-30; 215 °C meat ethyl ether/ acetonitrile gel diisopropyl ECD-GLC; N2; 0.001 90-95% at 0.01 5 petroleum permeation ether 40 ml/min; 1.8 m ether column SE-30 1% on gas (Styragel) Chrom. PAW milk hexane acetonitrile Florisil + benzene/ ECD-GLC; N2; 0.01 83-87% at 0.1 5 cellulose/ hexane 40 ml/min; 1.8 m charcoal (1/1) SE-30 1% on gas Chrom. PAW --------------------------------------------------------------------------------------------------------------------------------- Table 3. (contd.) ----------------------------------------------------------------------------------------------------------------------------------- Sample Extraction Sample preparation Determination: MDCb % Recovery Reference solvent ----------------------------------- (mg/kg) (fortification Partition Clean up GLC or HPLC level in column elution condition; detector, mg/kg) carrier flow, column, temp, R.T.a ----------------------------------------------------------------------------------------------------------------------------------- ENVIRONMENTAL ANALYSIS locust n-hexane Florisil ether/ ECD-GC; N2; 92 4 petroleum 75 ml/min; 0.6 m 5% ether (1/9) SE-30; 215 °C sea water XAD-2 ext.sol.c/ alumina ECD-GC; N2; 6 resin n-hexane 70 ml/min; 1.5 m acetone 4% SE-30; 207 °C water n-hexane alumina ECD-GC; N2; 6 70 ml/min; 1.5 m 4% SE-30; 207 °C water petroleum Florisil petroleum ECD-GLC; 1 m OV 0.0001 97 at 0.010 8 ether/ ether/ 1-3% on Chromosorb diethyl- diethyl- W.A.W. HMDS 60/80 ether (1/1) ether (80/20) soil acetone, acid hexane ECD-GLC; 5.2% 0.001 > 91% 9 acetone/ alumina ether OV-210 with AR/CH4 hexane (1/1) hexane hexane (5-10%) acetone, acid hexane/ ECD-GLC; N2; 0.0001 > 91% 5 acetone/ alumina ethyl ether 40 ml/min; 1.8 m hexane (1/1) (90/10) SE-30 1% on gas hexane Chrom. PAW cotton n-hexane transesterification 7 foliage followed by ECD-GC; (dislodgeable 31 ml/min; 0.45 m residue) 5% SE-30; 120 °C ----------------------------------------------------------------------------------------------------------------------------------- Table 3. (contd.) ----------------------------------------------------------------------------------------------------------------------------------- Sample Extraction Sample preparation Determination: MDCb % Recovery Reference solvent ----------------------------------- (mg/kg) (fortification Partition Clean up GLC or HPLC level in column elution condition; detector, mg/kg) carrier flow, column, temp, R.T.a ----------------------------------------------------------------------------------------------------------------------------------- PRODUCT ANALYSIS Technical HPLC, 230 nm; 15 cm 10 grade Lichrosorb Si-60; n-hexane/diisopropyl ether (93/7); 80 ml/h; 7.6 min isoctane/ HPLC - UV detector 5 dioxane 254 nm (230 nm for (80/20) conc. <0.5%) Silica-60; 100ml/h; isooctane/ dioxane (95/5) ----------------------------------------------------------------------------------------------------------------------------------- a R.T.: retention time; b MDC: minimum detectable concentration; c ext .sol.: extraction solvent. References 1. Baker & Bottomley (1982); 2. Mestres et al. (1978a); 3. Noble et al. (1982); 4. Pansu et al. (1981); 5. Vaysse et al. (1984); 6. Zitko et al. (1979); 7. Estesen et al. (1979); 8. Mestres et al. (1978b); 9. Hill (1982); 10. Mourot et al. (1979). 3. SOURCES OF ENVIRONMENTAL POLLUTION AND ENVIRONMENTAL LEVELS 3.1 Industrial Production Deltamethrin was first marketed in 1977. Production volumes in recent years are shown in Table 4. Table 4. Worldwide production of deltamethrin ------------------------------------------------- Year Production Reference (tonnes) ------------------------------------------------- 1979 75 Wood Mackenzie (1980) 1980 100 Wood Mackenzie (1981) 1981 100 Wood Mackenzie (1982, 1983) 1982 115 Wood Mackenzie (1983) 1987 250 Information from Roussel Uclaf -------------------------------------------------
Malaria control
Deltamethrin plays key role in controlling malaria vectors, and is used in the manufacture of long-lasting insecticidal mosquito nets. It is used as one of a battery of pyrethroid insecticides in control of malarial vectors, particularly Anopheles gambiae, and whilst being the most employed pyrethroid insecticide, can be used in conjunction with, or as an alternative to, permethrin, cypermethrin and other organophosphate-based insecticides, such as malathion and fenthion. Resistance to deltamethrin (and its counterparts) is now extremely widespread and threatens the success of worldwide vector control programmes.
Resistance to deltamethrin
Resistance has been characterised in several insects, including important vectors of malaria like the mosquito Anopheles gambiae as well as non-disease carrying pests like bed bugs.
Mosquitoes
Methods of resistance include thickening of the cuticle of the insect to limit permeation of the insecticide, metabolic resistance via overexpression of metabolising P450 mono-oxygenases and glutathione-S-transferases, and the knockdown resistance (kdr) sodium channel mutations which render the action of insecticides ineffectual, even when co-administered with piperonyl butoxide. Characterisation of the different forms of resistance among mosquitoes has become a top priority in groups studying tropical medicine due to the high mortality of those who reside in endemic areas.[3]
Bed Bugs
Two mutations, the Valine to Leucine mutation (V419L) and the Leucine to Isoleucine mutation (L925I) in voltage-gated sodium channel α-subunit gene, have been identified as responsible for knockdown resistance to deltamethrin in bed bugs. One study found that 88% of bed bug populations in the US had one, the other, or both mutations, meaning that deltamethrin resistance among bed bugs is currently making this insecticide obsolete.[4]
Poisoning
In humans
Since deltamethrin is a neurotoxin, it temporarily attacks (in medical terms, “insults”) the nervous system of any animal with which it comes into contact. Skin contact can lead to tingling or reddening of the skin local to the application. If taken in through the eyes or mouth, a common symptom is facial paraesthesia, which can feel like many different abnormal sensations, including burning, partial numbness, “pins and needles”, skin crawling, etc. There are no reports indicating that chronic intoxication from pyrethroid insecticides causes motor neuron damage or motor neuron disease.[5] However, in 2011, a case report was published that demonstrated pathologically proven motor-neuron death in a Japanese woman after acute massive ingestion of pesticides containing pyrethroids and organochlorine. [6]
Recently, in South Africa, residues of deltamethrin were found in breast milk, together with DDT, in an area that used DDT treatment for malaria control, as well as pyrethroids in small-scale agriculture.[7]
There are no antidotes, and treatment must be symptomatic, as approved by a physician. Over time, deltamethrin is metabolized, with a rapid loss of toxicity, and passed from the body. A poison control center should be contacted in the event of an accidental poisoning.
In domestic animals
Cases of toxicity have been observed in cattle, following use of agricultural deltamethrin preparation in external application in tick control. Symptoms appeared 36 hours after the application, and included muscular tremors that lead to decubitus 12 hours later. After 12 hours, there was spontaneous recovery and the animal could stand up again, although the muscular tremors persisted. The body temperature was then 38.3°C. (normal range 38.0 to 39.5°C.).
| Deltamethrin | |
|---|---|
 |
|
 |
|
| Identifiers | |
| CAS number | 52918-63-5 |
| ChemSpider | 37079 |
| UNII | 2JTS8R821G |
| KEGG | D07785 |
| ChEBI | CHEBI:4388 |
| ChEMBL | CHEMBL1593566 |
| ATC code | P03BA03,QP53AC11 |
| Jmol-3D images | Image 1 |
| Properties | |
| Molecular formula | C22H19Br2NO3 |
| Molar mass | 505.20 g mol−1 |
| Density | 1.5 g cm−3 |
| Melting point | 98 °C (208 °F; 371 K) |
| Boiling point | 300 °C (572 °F; 573 K) |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |
References
- “Deltamethrin Odorless Synthetic Pyrethroid Insecticides”. PestProducts.com. Retrieved 2008-09-26.
- Bouwman, B. Sereda and H.M. Meinhardt, H.; B. Sereda and H.M. Meinhardt (December 2006). “Simultaneous presence of DDT and pyrethroid residues in human breast milk from a malaria endemic area in South Africa”. Environmental Pollution 144 (3): 902–917. doi:10.1016/j.envpol.2006.02.002. PMID 16564119. Retrieved 2008-09-26.
- Muller, Pie, et al. (2008). Field caught Permethrin-Resistant Anopheles gambiae overexpress CYP6P3, a P450 that metabolises pyrethroids,PLoS Genetics 4(11)).
- Zhu, F.; Wigginton, J.; Romero, A.; Moore, A.; Ferguson, K.; Palli, R.; Potter, M. F.; Haynes, K. F.; Palli, S. R. (2010). “Widespread distribution of knockdown resistance mutations in the bed bug,Cimex lectularius(Hemiptera: Cimicidae), populations in the United States”. Archives of Insect Biochemistry and Physiology: n/a. doi:10.1002/arch.20355.
- Doi, et al (2006). “Motor neuron disorder simulating ALS induced by chronic inhalation of pyrethroid insecticides”. Neurology 67 (10). pp. 1894–1895.doi:10.1212/01.wnl.0000244489.65670.9f.
- Doi, et al (2006). “Motor neuron disorder simulating ALS induced by chronic inhalation of pyrethroid insecticides”. Neurology 67 (10). pp. 1894–1895.doi:10.1212/01.wnl.0000244489.65670.9f.
- Bouwman, B. Sereda and H.M. Meinhardt, H.; B. Sereda and H.M. Meinhardt (December 2006). “Simultaneous presence of DDT and pyrethroid residues in human breast milk from a malaria endemic area in South Africa”. Environmental Pollution 144 (3): 902–917. doi:10.1016/j.envpol.2006.02.002. PMID 16564119. Retrieved 2008-09-26.
External links
- Deltamethrin General Fact Sheet – National Pesticide Information Center
- Deltamethrin Technical Fact Sheet – National Pesticide Information Center
- Data Sheet on Pesticides No. 50 – IPCS INCHEM
- Pyrethrins and Pyrethroids Fact Sheet – National Pesticide Information Center
- Deltamethrin Pesticide Information Profile – Extension Toxicology Network
Literature References:
Synthetic pyrethroid insecticide. Prepn of racemic mixture: M. Elliot et al., DE 2439177 (1975 to NRDC),C.A. 83, 73519z (1975); of decamethrin and isomers: eidem,
Pestic. Sci. 9, 105 (1978). Activity: eidem, Nature 248, 710 (1974);eidem, Pestic. Sci. 9, 112 (1978).
Absolute configuration: J. D. Owen, J. Chem. Soc. Perkin Trans. 1 1975, 1865.
Photochemistry: L. O. Ruzo et al., J. Agric. Food Chem. 25, 1385 (1977).
Metabolism: eidem, ibid. 26, 918 (1978). Toxicology: R. Kavlock et al., J. Environ. Pathol. Toxicol. 2, 751 (1979).
Pharmacological effects on central nervous system: P. H. Chanh et al.,Arzneim.-Forsch. 34, 175 (1984).
Review of toxicology and human exposure: Toxicological Profile for Pyrethrins and Pyrethroids(PB2004-100004, 2003) 332 pp.
file:///C:/Users/Inspiron/Downloads/molecules-17-13989-v2.pdf
|
6-19-1985
|
Stabilized mixtures of carbamate insecticides and synthetic pyrethroids
|
|
|
8-8-1984
|
Pesticides
|
|
|
10-20-1983
|
Stabilized mixtures of carbamate insecticides and synthetic pyrethroids. STABILIZED MIXTURES OF CARBAMATE INSECTICIDES AND SYNTHETIC PYRETHROIDS
|
|
|
11-11-1981
|
Stabilized cyandhydrin ester
|
|
|
7-23-1980
|
Conversion of stereoisomer into its diastereoisomer
|















Sorry, the comment form is closed at this time.