
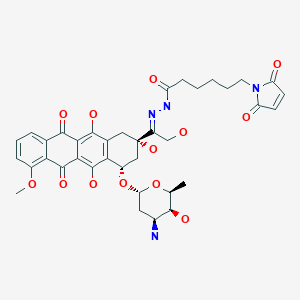
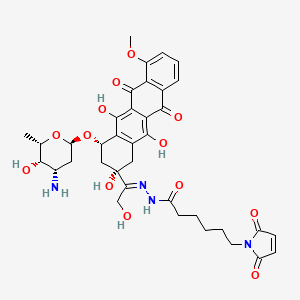
Aldoxorubicin, DOXO-EMCH
N’-[1-[4(S)-(3-Amino-2,3,6-trideoxy-alpha-L-lyxo-hexopyranosyloxy)-2(S),5,12-trihydroxy-7-methoxy-6,11-dioxo-1,2,3,4,6,11-hexahydronaphthacen-2-yl]-2-hydroxyethylidene]-6-(2,5-dioxo-2,5-dihydro-1H-pyrrol-1-yl)hexanohydrazide
1H-Pyrrole-1-hexanoic acid, 2,5-dihydro-2,5-dioxo-, (2E)-2-[1-[(2S,4S)-4-[(3-amino-2,3,6-trideoxy-α-L-lyxo– hexopyranosyl)oxy]-1,2,3,4,6,11-hexahydro-2,5,12- trihydroxy-7-methoxy-6,11-dioxo-2-naphthacenyl]-2- hydroxyethylidene]hydrazide
CytRx is pouring money into R&D of cancer-fighting drugs see article
Los Angeles Times
s most promising cancer-fighting drug, aldoxorubicin, is “sort of like a guided … Phase 3 clinical trial of a second-line treatment for soft-tissue sarcoma.

Aldoxorubicin
http://www.ama-assn.org/resources/doc/usan/aldoxorubicin.pdf
 in phase 3 Cytrx Corporation
in phase 3 Cytrx Corporation
(E)-N’-(1-((2S,4S)-4-(((2R,4S,5S,6S)-4-amino-5-hydroxy-6-methyltetrahydro-2H-pyran-2-yl)oxy)-2,5,12-trihydroxy-7-methoxy-6,11-dioxo-1,2,3,4,6,11-hexahydrotetracen-2-yl)-2-hydroxyethylidene)-6-(2,5-dioxo-2,5-dihydro-1H-pyrrol-1-yl)hexanehydrazide hydrochloride
1H-Pyrrole-1-hexanoic acid, 2,5-dihydro-2,5-dioxo-, (2E)-2-[1-[(2S,4S)-4-[(3-amino-
2,3,6-trideoxy-α-L-lyxo-hexopyranosyl)oxy]-1,2,3,4,6,11-hexahydro-2,5,12-trihydroxy-
7-methoxy-6,11-dioxo-2-naphthacenyl]-2-hydroxyethylidene]hydrazide
N’-[(1E)-1-{(2S,4S)-4-[(3-amino-2,3,6-trideoxy-α-L-lyxo-hexopyranosyl)oxy]-2,5,12-
trihydroxy-7-methoxy-6,11-dioxo-1,2,3,4,6,11-hexahydrotetracen-2-yl}-2-
hydroxyethylidene]-6-(2,5-dioxo-2,5-dihydro-1H-pyrrol-1-yl)hexanohydrazide
MOLECULAR FORMULA C37H42N4O13
MOLECULAR WEIGHT 750.7
SPONSOR CytRx Corp.
CODE DESIGNATION
- Aldoxorubicin
- INNO 206
- INNO-206
- UNII-C28MV4IM0B
CAS REGISTRY NUMBER 1361644-26-9
CAS: 151038-96-9 (INNO-206); 480998-12-7 (INNO-206 HCl salt), 1361644-26-9
| QC data: | |
| Safety Data Sheet (MSDS): |

hydrochloride
CAS: 151038-96-9
Chemical Formula: C37H42N4O13
Exact Mass: 750.27484
Molecular Weight: 750.75
| Certificate of Analysis: | |
| QC data: | |
| Safety Data Sheet (MSDS): |

| In vitro protocol: | Clin Cancer Res. 2012 Jul 15;18(14):3856-67 |
| In vivo protocol: | Clin Cancer Res. 2012 Jul 15;18(14):3856-67.Invest New Drugs. 2010 Feb;28(1):14-9.Invest New Drugs. 2012 Aug;30(4):1743-9.Int J Cancer. 2007 Feb 15;120(4):927-34. |
| Clinical study: | Expert Opin Investig Drugs. 2007 Jun;16(6):855-66. |

Aldoxorubicin (INNO-206): Aldoxorubicin, also known as INNO-206, is the 6-maleimidocaproyl hydrazone derivative prodrug of the anthracycline antibiotic doxorubicin (DOXO-EMCH) with antineoplastic activity. Following intravenous administration, doxorubicin prodrug INNO-206 binds selectively to the cysteine-34 position of albumin via its maleimide moiety. Doxorubicin is released from the albumin carrier after cleavage of the acid-sensitive hydrazone linker within the acidic environment of tumors and, once located intracellularly, intercalates DNA, inhibits DNA synthesis, and induces apoptosis. Albumin tends to accumulate in solid tumors as a result of high metabolic turnover, rapid angiogenesis, hyervasculature, and impaired lymphatic drainage. Because of passive accumulation within tumors, this agent may improve the therapeutic effects of doxorubicin while minimizing systemic toxicity.
“Aldoxorubicin has demonstrated effectiveness against a range of tumors in both human and animal studies, thus we are optimistic in regard to a potential treatment for Kaposi’s sarcoma. The current standard-of-care for severe dermatological and systemic KS is liposomal doxorubicin (Doxil®). However, many patients exhibit minimal to no clinical response to this agent, and that drug has significant toxicity and manufacturing issues,” said CytRx President and CEO Steven A. Kriegsman. “In addition to obtaining valuable information related to Kaposi’s sarcoma, this trial represents another opportunity to validate the value and viability of our linker technology platform.” The company expects to announce Phase-2 study results in the second quarter of 2015.
Kaposi’s sarcoma is an orphan indication, meaning that only a small portion of the population has been diagnosed with the disease (fewer than 200,000 individuals in the country), and in turn, little research and drug development is being conducted to treat and cure it. The FDA’s Orphan Drug Act may grant orphan drug designation to a drug such as aldoxorubicin that treats a rare disease like Kaposi’s sarcoma, offering market exclusivity for seven years, fast-track status in some cases, tax credits, and grant monies to accelerate research
The widely used chemotherapeutic agent doxorubicin is delivered systemically and is highly toxic, which limits its dose to a level below its maximum therapeutic benefit. Doxorubicin also is associated with many side effects, especially the potential for damage to heart muscle at cumulative doses greater than 450 mg/m2. Aldoxorubicin combines doxorubicin with a novel single-molecule linker that binds directly and specifically to circulating albumin, the most plentiful protein in the bloodstream. Protein-hungry tumors concentrate albumin, thus increasing the delivery of the linker molecule with the attached doxorubicin to tumor sites. In the acidic environment of the tumor, but not the neutral environment of healthy tissues, doxorubicin is released. This allows for greater doses (3 1/2 to 4 times) of doxorubicin to be administered while reducing its toxic side effects. In studies thus far there has been no evidence of clinically significant effects of aldoxorubicin on heart muscle, even at cumulative doses of drug well in excess of 2,000 mg/m2.
INNO-206 is an anthracycline in early clinical trials at CytRx Oncology for the treatment of breast cancer, HIV-related Kaposi’s sarcoma, glioblastoma multiforme, stomach cancer and pancreatic cancer. In 2014, a pivotal global phase 3 clinical trial was initiated as second-line treatment in patients with metastatic, locally advanced or unresectable soft tissue sarcomas. The drug candidate was originally developed at Bristol-Myers Squibb, and was subsequently licensed to KTB Tumorforschungs. In August 2006, Innovive Pharmaceuticals (acquired by CytRx in 2008) licensed the patent rights from KTB for the worldwide development and commercialization of the drug candidate. No recent development has been reported for research that had been ongoing for the treatment of small cell lung cancer (SCLC).
INNO-206 is a doxorubicin prodrug. Specifically, it is the 6-maleimidocaproyl hydrazone of doxorubicin. After administration, the drug candidate rapidly binds endogenous circulating albumin through the acid sensitive EMCH linker. Circulating albumin preferentially accumulates in tumors, bypassing uptake by other non-specific sites including the heart, bone marrow and the gastrointestinal tract. Once inside the acidic environment of the tumor cell, the EMCH linker is cleaved and free doxorubicin is released at the tumor site. Like other anthracyclines, doxorubicin inhibits DNA and RNA synthesis by intercalating between base pairs of the DNA/RNA strand, thus preventing the replication of rapidly-growing cancer cells. It also creates iron-mediated free oxygen radicals that damage the DNA and cell membranes. In 2011, orphan drug designation was assigned in the U.S. for the treatment of pancreatic cancer and for the treatment of soft tissue sarcoma.
CytRx Corporation (NASDAQ:CYTR) has announced it has initiated a pivotal global Phase 3 clinical trial to evaluate the efficacy and safety of aldoxorubicin as a second-line treatment for patients with soft tissue sarcoma (STS) under a Special Protocol Assessment with the FDA. Aldoxorubicin combines the chemotherapeutic agent doxorubicin with a novel linker-molecule that binds specifically to albumin in the blood to allow for delivery of higher amounts of doxorubicin (3.5 to 4 times) without several of the major treatment-limiting toxicities seen with administration of doxorubicin alone.
According to a news from Medicalnewstoday.com; CytRx holds the exclusive worldwide rights to INNO-206. The Company has previously announced plans to initiate Phase 2 proof-of-concept clinical trials in patients with pancreatic cancer, gastric cancer and soft tissue sarcomas, upon the completion of optimizing the formulation of INNO-206. Based on the multiple myeloma interim results, the Company is exploring the possibility of rapidly including multiple myeloma in its INNO-206 clinical development plans.
According to CytRx’s website, In preclinical models, INNO-206 was superior to doxorubicin with regard to ability to increase dosing, antitumor efficacy and safety. A Phase I study of INNO-206 that demonstrated safety and objective clinical responses in a variety of tumor types was completed in the beginning of 2006 and presented at the March 2006 Krebskongress meeting in Berlin. In this study, doses were administered at up to 4 times the standard dosing of doxorubicin without an increase in observed side effects over historically seen levels. Objective clinical responses were seen in patients with sarcoma, breast, and lung cancers.
INNO-206 – Mechanism of action:
According to CytRx’s website, the proposed mechanism of action is as the follow steps: (1) after administration, INNO-206 rapidly binds endogenous circulating albumin through the EMCH linker. (2) circulating albumin preferentially accumulates in tumors, bypassing uptake by other non-specific sites including heart, bone marrow and gastrointestinal tract; (3) once albumin-bound INNO-206 reaches the tumor, the acidic environment of the tumor causes cleavage of the acid sensitive linker; (4) free doxorubicin is released at the site of the tumor.
INNO-206 – status of clinical trials:
CytRx has announced that, in December 2011, CytRx initiated its international Phase 2b clinical trial to evaluate the preliminary efficacy and safety of INNO-206 as a first-line therapy in patients with soft tissue sarcoma who are ineligible for surgery. The Phase 2b clinical trial will provide the first direct clinical trial comparison of INNO-206 with native doxorubicin, which is dose-limited due to toxicity, as a first-line therapy. (source:http://cytrx.com/inno_206, accessed date: 02/01/2012).
Results of Phase I study:
In a phase I study a starting dose of 20 mg/m2 doxorubicin equivalents was chosen and 41 patients with advanced cancer disease were treated at dose levels of 20–340 mg/m2 doxorubicin equivalents . Treatment with INNO-206 was well tolerated up to 200 mg/m2 without manifestation of drug-related side effects which is a ~3-fold increase over the standard dose for doxorubicin (60 mg/kg). Myelosuppression and mucositis were the predominant adverse effects at dose levels of 260 mg/m2 and became dose-limiting at 340 mg/m2. 30 of 41 patients were assessable for analysis of response. Partial responses were observed in 3 patients (10%, small cell lung cancer, liposacoma and breast carcinoma). 15 patients (50%) showed a stable disease at different dose levels and 12 patients (40%) had evidence of tumor progression. (source: Invest New Drugs (2010) 28:14–19)
phase 2
CytRx Corporation (CYTR), a biopharmaceutical research and development company specializing in oncology, today announced that its oral presentation given by Sant P. Chawla, M.D., F.R.A.C.P., Director of the Sarcoma Oncology Center, titled “Randomized phase 2b trial comparing first-line treatment with aldoxorubicin versus doxorubicin in patients with advanced soft tissue sarcomas,” was featured in The Lancet Oncology in its July 2014 issue (Volume 15, Issue 8) in a review of the major presentations from the 2014 American Society of Clinical Oncology (ASCO) Annual Meeting.
“We are honored to have been included in The Lancet Oncology’s review of major presentations from ASCO and pleased that these important clinical findings are being recognized by one of the world’s premier oncology journals,” said Steven A. Kriegsman, CytRx President and CEO. “In clinical trials, aldoxorubicin has been shown to be a well-tolerated and efficacious single agent for the treatment of soft tissue sarcoma (STS) that lacks the cardiotoxicity associated with doxorubicin therapy, the current standard of care. We remain on track to report the full overall survival results from this trial prior to year-end 2014.”
The data presented at ASCO 2014 were updated results from CytRx’s ongoing multicenter, randomized, open-label global Phase 2b clinical trial investigating the efficacy and safety of aldoxorubicin compared with doxorubicin as first-line therapy in subjects with metastatic, locally advanced or unresectable STS. The updated trial results demonstrated that aldoxorubicin significantly increases progression-free survival (PFS), PFS at 6 months, overall response rate (ORR) and tumor shrinkage, compared to doxorubicin, the current standard-of-care, as a first-line treatment in patients with STS. The data trended in favor of aldoxorubicin for all of the major subtypes of STS
phase 3
Aldoxorubicin is currently being studied in a pivotal global Phase 3 clinical trial evaluating the efficacy and safety of aldoxorubicin as a second-line treatment for patients with STS under a Special Protocol Assessment with the FDA. CytRx is also conducting two Phase 2 clinical trials evaluating aldoxorubicin in patients with late-stage glioblastoma (GBM) and HIV-related Kaposi’s sarcoma and expects to start a phase 2b study in patients with relapsed small cell lung cancer
PATENTS WO 2000076551, WO 2008138646, WO 2011131314,
…………………….
WO 2014093815
http://www.google.com/patents/WO2014093815A1?cl=en
Anthracyclines are a class of antibiotics derived from certain types of Streptomyces bacteria. Anthracyclines are often used as cancer therapeutics and function in part as nucleic acid intercalating agents and inhibitors of the DNA repair enzyme topoisomerase II, thereby damaging nucleic acids in cancer cells, preventing the cells from replicating. One example of an anthracycline cancer therapeutic is doxorubicin, which is used to treat a variety of cancers including breast cancer, lung cancer, ovarian cancer, lymphoma, and leukemia. The 6-maleimidocaproyl hydrazone of doxorubicin (DOXO-EMCH) was originally synthesized to provide an acid-sensitive linker that could be used to prepare immunoconjugates of doxorubicin and monoclonal antibodies directed against tumor antigens (Willner et al., Bioconjugate Chem 4:521-527 (1993)). In this context, antibody disulfide bonds are reduced with dithiothreitol to form free thiol groups, which in turn react with the maleimide group of DOXO-EMCH to form a stable thioether bond. When administered, the doxorubicin-antibody conjugate is targeted to tumors containing the antigen recognized by the antibody. Following antigen-antibody binding, the conjugate is internalized within the tumor cell and transported to lysosomes. In the acidic lysosomal environment, doxorubicin is released from the conjugate intracellularly by hydrolysis of the acid-sensitive hydrazone linker. Upon release, the doxorubicin reaches the cell nucleus and is able to kill the tumor cell. For additional description of doxorubicin and
DOXO-EMCH see, for example, U.S. Patents 7,387,771 and 7,902,144 and U.S. Patent Application No. 12/619,161, each of which are incorporated in their entirety herein by reference.
[0003] A subsequent use of DOXO-EMCH was developed by reacting the molecule in vitro with the free thiol group (Cys-34) on human serum albumin (HSA) to form a stable thioether conjugate with this circulating protein (Kratz et al, J Med Chem 45:5523-5533 (2002)). Based on these results, it was
hypothesized that intravenously-administered DOXO-EMCH would rapidly conjugate to HSA in vivo and that this macromolecular conjugate would preferentially accumulate in tumors due to an “enhanced permeability and retention” (EPR) intratumor effect (Maeda et al., J Control Release 65:271-284 (2000)).
[0004] Acute and repeat-dose toxicology studies with DOXO-EMCH in mice, rats, and dogs identified no toxicity beyond that associated with doxorubicin, and showed that all three species had significantly higher tolerance for DOXO-EMCH compared to doxorubicin (Kratz et al, Hum Exp Toxicol 26: 19-35 (2007)). Based on the favorable toxicology profile and positive results from animal tumor models, a Phase 1 clinical trial of DOXO-EMCH was conducted in 41 advanced cancer patients (Unger et al, Clin Cancer Res 13:4858-4866 (2007)). This trial found DOXO-EMCH to be safe for clinical use. In some cases, DOXO-EMCH induced tumor regression.
[0005] Due to the sensitivity of the acid-cleavable linker in DOXO-EMCH, it is desirable to have formulations that are stable in long-term storage and during reconstitution (of, e.g., previously lyophilized compositions) and administration. DOXO-EMCH, when present in compositions, diluents and administration fluids used in current formulations, is stable only when kept at low temperatures. The need to maintain DOXO-EMCH at such temperatures presents a major problem in that it forces physicians to administer cold (4°C) DOXO-EMCH compositions to patients. Maintaining DOXO-EMCH at low temperatures complicates its administration in that it requires DOXO-EMCH to be kept at 4°C and diluted at 4°C to prevent degradation that would render it unsuitable for patient use. Further, administration at 4°C can be harmful to patients whose body temperature is significantly higher (37°C).
[0006] Lyophilization has been used to provide a stable formulation for many drugs. However, reconstitution of lyophilized DOXO-EMCH in a liquid that does not maintain stability at room temperature can result in rapid decomposition of DOXO-EMCH. Use of an inappropriate diluent to produce an injectable composition of DOXO-EMCH can lead to decreased stability and/or solubility. This decreased stability manifests itself in the cleavage of the linker between the doxorubicin and EMCH moieties, resulting in degradation of the DOXO-EMCH into two components: doxorubicin and linker-maleimide. Thus, stable,
reconstituted lyophilized solutions of anthracycline-EMCH (e.g., DOXO-EMCH), and injectable compositions containing the same, are required to solve these problems and to provide a suitable administration vehicle that can be used reasonably in treating patients both for clinical trials and commercially.
DOXO-EMCH. The term “DOXO-EMCH,” alone or in combination with any other term, refers to a compound as depicted by the following structure:
OH
DOXO-EMCH is also referred to as (E)-N’-(l-((2S,4S)-4-(4-amino-5-hydroxy-6- methyl-tetrahydro-2H-pyran-2-yloxy-2,5 , 12-trihydroxy-7-methoxy-6, 11- dioxol,2,3,4,6,l l-hexahydrotetracen-2-yl)-2-hydroxyethylidene)-6-(2,5-dioxo-2H- pyrrol- 1 (5H)yl)hexanehydrazide»HCl.
………………………………
CN 102675385
http://www.google.com/patents/CN102675385A?cl=en
According to literature reports, (eg see David Willner et al, “(6_Maleimidocaproyl) hydrazoneof Doxorubicm-A New Derivative for the Preparation ofImmunoconjugates oiDoxorubicin,” Bioconjugate Chem. 1993,4, 521-527; JK Tota Hill, etc. man, “The method of preparation of thioether compounds noir,” CN1109886A, etc.), adriamycin 13 – bit hydrazone derivative synthesis and the main process are as follows:
[0004]
[0005] First, maleic anhydride and 6 – aminocaproic acid was refluxed in a large number of acid reaction ko ni acid I; agent under the action of the ring after the cyclization maleimidocaproic acid 2 (yield 30-40% ), cyclic acid anhydride mixture is generally ko, trimethyl silyl chloride and tri-amines such ko; maleimido aminocaproic acid tert-butyl ester with hydrazine to condensation to give 2 – (6 – aminocaproic maleimido ) hydrazine carboxylic acid tert-butyl ester 3 (yield 70-85%), the condensing agent is N-methylmorpholine and isobutyl chloroformate; 3 in a large number of trifluoroacetic acid deprotection ko maleimido ko has trifluoroacetic acid hydrazide 4 (yield 70%); the doxorubicin hydrochloride salt with a ko in trifluoroacetic acid catalyzed condensation in methanol solvent to doxorubicin hydrazone product was obtained (yield 80%) .
[0006] The synthetic method the yield is low (in particular, by maleic acid imido step 2), the total yield of not more than 20%, and the solvent consumption is large, adriamycin hydrazone product per Malek consumes about ko acid reaction solvent, 70mL, tetrahydrofuran 300mL, ko trifluoroacetic acid 40mL, and because the 2 – (6 – maleimido hexanoyl)-hydrazine carboxylic acid tert-butyl ester was purified by column chromatography required, but also to consume a large amount of Solvent. This has resulted in synthesis post-processing complex process, complicated operation. And because the end product of the synthesis of doxorubicin hydrazone ko using trifluoroacetic acid, inevitably there will be in the product ko trifluoroacetic acid impurities, not divisible. Based on the high cost of such a route exists, yield and production efficiency is low, Eri Arts route operational complexity and other shortcomings, is obviously not suitable for mass production, it is necessary to carry out improvements or exploring other Eri Arts synthesis methods.
doxorubicin hydrazone derivative,
Wherein n is an integer of 1-15, characterized in that said method comprises the steps of: (1) the maleic acid chloride of the formula H2N-(CH2) n-COOH amino acid I b in the presence of a base prepared by condensation of maleimido group steps I c acid,
(2) maleic acid imido group I c and then with an acylating reagent of tert-butyl carbazate in the presence of a base in the reaction of step I d,
(3) I d deprotection with trifluoroacetic acid, the alkali and removing trifluoroacetic acid to obtain the maleimido group I e hydrazide steps
(4) an imido group of maleic hydrazide I e and doxorubicin hydrochloride catalyzed condensation of hydrogen chloride to obtain a final product hydrazone derivative of doxorubicin,
[0028]
[0049] The butene-ni chloride 15. 2g (0. Imol) was dissolved in 25mL of chloroform was dried by adding anhydrous potassium carbonate 27. 6g (0. 2mol), the gas and gas protection and conditions of 0 ° C was added dropwise 6 – aminocaproic acid 13. 2g (0. ImoI) in chloroform (50mL) solution, add after reaction at room temperature for 3 hours. Washed with saturated brine, dried over anhydrous magnesium sulfate, suction filtered, concentrated under reduced pressure. The residue was recrystallized from alcohol ko maleimido acid (Compound c) 18. 8g, 90% yield, m.p. :85-87 ° C.
[0050] Compound c 10. 5g (50mmol) and thionyl chloride crab 5. 3mL (75mmol) was heated under reflux the mixture I. 5 hours and concentrated under reduced pressure in an argon atmosphere under the conditions of 0 ° C and added dropwise to the hydrazine carboxylic acid tert-butyl ester 6.6g (50mmol) amine with a three ko
10. 8mL (75mmol) in anhydrous ko ether (50mL) solution added after the reaction was continued at room temperature for I. 5 hours. Washed with 5% hydrochloric acid, 5% sodium bicarbonate, and saturated brine, dried over anhydrous magnesium sulfate overnight, filtered with suction to give the compound of d ko ether solution. The solution was cooled to 0 ° C, was added dropwise trifluoroacetic acid ko 7. 4mL (100mmOl), After the addition the reaction was continued for 10 minutes, suction filtered, the filter cake was washed twice with ether, ko and dried in vacuo to give 6 – maleic acid sub-aminocaproic acid hydrazide trifluoro-ko 12. 2g, 72% yield, m.p. 99-102 ° C. IOmL this salt is added to sodium hydroxide (10%) solution, stirred for a while, with ko extracted with ether, the organic layer was washed with water, dried over anhydrous magnesium sulfate, and concentrated to give 6 – aminocaproic maleimido hydrazide (compound e) 7. Sg, 70% yield.
[0051] The doxorubicin hydrochloride 0. 58g (Immol) with compound e 0. 45g (2mmol) was dissolved in 150mL of anhydrous methanol, passing about 2mmol of dry hydrogen chloride, under argon, at room temperature protected from light and reaction conditions 24 inches. Concentrated under reduced pressure at room temperature, the solid was washed with about IOOmL ko nitrile, and dried in vacuo doxorubicin 6 – aminocaproic maleimido hydrazone O. 63g, 80% yield. 1H NMR (CD3OD) δ: 7. 94 (bd, 1H), 7. 82 (t, 1H), 7. 55 (d, 1H), 6. 78 (s, 2H), 5. 48 (s, 1H ), 5. 07 (t, 1H), 4 · 59 (d, 1H), 4 · 21 (m, 1Η), 4 · 02 (s, 3H), 3 · 63-3. 30 (m, 5H) , 2 · 55-2. 26 (m, 4H), 2. 19-1. 88 (m, 3Η), I. 69-1. 18 (m, 12Η, I. 26). [0052] Although specific reference to the above embodiments of the present invention will be described, it will be understood that in the appended claims without departing from the invention as defined by the spirit and scope of the skilled person can be variously truncated, substitutions and changes. Accordingly, the present invention encompasses these deletions, substitutions and changes.
………………………………….
US 5622929
http://www.google.co.in/patents/US5622929
http://www.google.co.in/patents/EP0554708A1
Method A:
As noted below, Method A is the preferred method when the Michael Addition Receptor is a maleimido moiety.
Alternatively, the Formula (IIa) compound may be prepared by reaction of the drug with a hydrazide to form an intermediate hydrazone drug derivative followed by reaction of this compound with a Michael Addition Receptor containing moiety according to the general process described in Method B:
…………………………………….
http://www.google.co.in/patents/WO2012167255A1?cl=en
Synthesis of DOXO-EMCH
The synthesis of DOXO-EMCH was done initially in accordance with that previously published by Willner and co-workers (Bioconjugate Chem., 4:521-527, 1993). Problems arose in the initial addition of the 6-maleimidocaproylhydrazine to the C-13 ketone of doxorubicin. HPLC results did not give a good yield of product, only 50-60%. Upon further analysis, we determined TFA was not needed to catalyze the reaction, and instead used pyridine. With pyridine, chromatograms from the HPLC showed 90% DOXO-EMCH relative to 10% DOX. The pyridine may have improved the yield by serving as a base to facilitate formation of the hydrazone. Another problem we encountered in the synthesis was purification of the final product. According to Willner’ s method, 5 volumes of acetonitrile (ACN) were to be added to a concentrated methanolic solution of crude DOXO-EMCH to achieve crystallization after 48 h at 4 °C. By this method, only 10-20%) of the desired product precipitated. To obtain a better yield, the crystallization step was done 4 times with 6 volumes of ACN used in each step. A lesser amount of methanol was needed each time, as less product remained in solution. Even with the multiple crystallizations, a final yield of only 59% was obtained. Various other methods for crystallization were explored, including using different solvents and increasing the initial solubility in methanol by heat, but none gave better results. 1.2 Rate of Hydrolysis of DOXO-EMCH at Varying pH
Subsequent pH studies to determine the rate of hydrolysis of the hydrazone were carried out as a benchmark for later hydrolysis experiments with PPD-EMCH. The results of the hydrolysis experiments showed that at lower pH, the hydrolysis reaction proceeded very quickly in the formation of DOX. Moreover, at higher pH the hydrazone proved to be very robust in that its degradation is very slow.
General HPLC instruments and methods
Analytical HPLC methods were performed using a Hewlett-Packard/ Aligent 1050/1100 chromatograph with an auto injector, diode array UV-vis absorption detector. Method 1.1 : Analytical HPLC injections were onto an Aligent Zorbax Eclipse XDB-C18 reversed phase column, 4.6 mm x 150 mm, eluting at 1.0 mL/min. A gradient of acetonitrile/20 mM sodium phosphate buffer (pH 6.9), 80% buffer to 55% at 10 min, 55% to 40% at 12 min, 40% to 80% at 13 min. Retention times: at 480 nm, DOX (9.4 min), DOXO-EMCH (1 1.2 min).
Synthesis of DOXO-EMCH
The synthesis of DOXO-EMCH was accomplished using the procedure reported by Willner et al, with several changes to improve the yield (Willner, D., et al.,
Bioconjugate Chem., 4:521-27, 1993). DOX’HCl (20 mg, 34 μιηοΐ) was dissolved in 6 mL of methanol. Pyridine (12.53 μί) was added to the solution, followed by 35.4 mg
EMCH’TFA. The reaction was stirred at room temperature overnight. By HPLC, the reaction was 90% complete. The solvent was evaporated to dryness by rotary evaporation. A minimal amount of methanol was used to dissolve the solid, and six volumes of acetonitrile at 4 °C were added to the solution. The resulting solution was allowed to sit undisturbed at 4 °C for 48 h for crystallization. The precipitate was collected, and the crystallization method was repeated 4 times. The resulting solids were combined and washed three times with 1 : 10 methanokacetonitrile. The final yield of DOXO-EMCH was 11.59 mg, 58%. HPLC Method 1.1 was used. NMR spectra corresponded to those previously given by Willner (Bioconjugate Chem. 4:521-27. 1993).
…………………………….
http://www.google.co.in/patents/US20070219351
DOXO-EMCH, the structural formula of which is shown below,
…………………………………
SEE
(6-Maleimidocaproyl)hydrazone of doxorubicin – A new derivative for the preparation of immunoconjugates of doxorubicin
Bioconjugate Chem 1993, 4(6): 521
| References |
1: Kratz F, Azab S, Zeisig R, Fichtner I, Warnecke A. Evaluation of combination therapy schedules of doxorubicin and an acid-sensitive albumin-binding prodrug of doxorubicin in the MIA PaCa-2 pancreatic xenograft model. Int J Pharm. 2013 Jan 30;441(1-2):499-506. doi: 10.1016/j.ijpharm.2012.11.003. Epub 2012 Nov 10. PubMed PMID: 23149257.
2: Walker L, Perkins E, Kratz F, Raucher D. Cell penetrating peptides fused to a thermally targeted biopolymer drug carrier improve the delivery and antitumor efficacy of an acid-sensitive doxorubicin derivative. Int J Pharm. 2012 Oct 15;436(1-2):825-32. doi: 10.1016/j.ijpharm.2012.07.043. Epub 2012 Jul 28. PubMed PMID: 22850291; PubMed Central PMCID: PMC3465682.
3: Kratz F, Warnecke A. Finding the optimal balance: challenges of improving conventional cancer chemotherapy using suitable combinations with nano-sized drug delivery systems. J Control Release. 2012 Dec 10;164(2):221-35. doi: 10.1016/j.jconrel.2012.05.045. Epub 2012 Jun 13. PubMed PMID: 22705248.
4: Sanchez E, Li M, Wang C, Nichols CM, Li J, Chen H, Berenson JR. Anti-myeloma effects of the novel anthracycline derivative INNO-206. Clin Cancer Res. 2012 Jul 15;18(14):3856-67. doi: 10.1158/1078-0432.CCR-11-3130. Epub 2012 May 22. PubMed PMID: 22619306.
5: Kratz F, Elsadek B. Clinical impact of serum proteins on drug delivery. J Control Release. 2012 Jul 20;161(2):429-45. doi: 10.1016/j.jconrel.2011.11.028. Epub 2011 Dec 1. Review. PubMed PMID: 22155554.
6: Elsadek B, Kratz F. Impact of albumin on drug delivery–new applications on the horizon. J Control Release. 2012 Jan 10;157(1):4-28. doi: 10.1016/j.jconrel.2011.09.069. Epub 2011 Sep 16. Review. PubMed PMID: 21959118.
7: Kratz F, Fichtner I, Graeser R. Combination therapy with the albumin-binding prodrug of doxorubicin (INNO-206) and doxorubicin achieves complete remissions and improves tolerability in an ovarian A2780 xenograft model. Invest New Drugs. 2012 Aug;30(4):1743-9. doi: 10.1007/s10637-011-9686-5. Epub 2011 May 18. PubMed PMID: 21590366.
8: Boga C, Fiume L, Baglioni M, Bertucci C, Farina C, Kratz F, Manerba M, Naldi M, Di Stefano G. Characterisation of the conjugate of the (6-maleimidocaproyl)hydrazone derivative of doxorubicin with lactosaminated human albumin by 13C NMR spectroscopy. Eur J Pharm Sci. 2009 Oct 8;38(3):262-9. doi: 10.1016/j.ejps.2009.08.001. Epub 2009 Aug 18. PubMed PMID: 19695327.
9: Graeser R, Esser N, Unger H, Fichtner I, Zhu A, Unger C, Kratz F. INNO-206, the (6-maleimidocaproyl hydrazone derivative of doxorubicin), shows superior antitumor efficacy compared to doxorubicin in different tumor xenograft models and in an orthotopic pancreas carcinoma model. Invest New Drugs. 2010 Feb;28(1):14-9. doi: 10.1007/s10637-008-9208-2. Epub 2009 Jan 8. PubMed PMID: 19148580.
10: Kratz F. Albumin as a drug carrier: design of prodrugs, drug conjugates and nanoparticles. J Control Release. 2008 Dec 18;132(3):171-83. doi: 10.1016/j.jconrel.2008.05.010. Epub 2008 May 17. Review. PubMed PMID: 18582981.
11: Unger C, Häring B, Medinger M, Drevs J, Steinbild S, Kratz F, Mross K. Phase I and pharmacokinetic study of the (6-maleimidocaproyl)hydrazone derivative of doxorubicin. Clin Cancer Res. 2007 Aug 15;13(16):4858-66. PubMed PMID: 17699865.
12: Lebrecht D, Walker UA. Role of mtDNA lesions in anthracycline cardiotoxicity. Cardiovasc Toxicol. 2007;7(2):108-13. Review. PubMed PMID: 17652814.
13: Kratz F. DOXO-EMCH (INNO-206): the first albumin-binding prodrug of doxorubicin to enter clinical trials. Expert Opin Investig Drugs. 2007 Jun;16(6):855-66. Review. PubMed PMID: 17501697.
14: Kratz F, Ehling G, Kauffmann HM, Unger C. Acute and repeat-dose toxicity studies of the (6-maleimidocaproyl)hydrazone derivative of doxorubicin (DOXO-EMCH), an albumin-binding prodrug of the anticancer agent doxorubicin. Hum Exp Toxicol. 2007 Jan;26(1):19-35. PubMed PMID: 17334177.
15: Lebrecht D, Geist A, Ketelsen UP, Haberstroh J, Setzer B, Kratz F, Walker UA. The 6-maleimidocaproyl hydrazone derivative of doxorubicin (DOXO-EMCH) is superior to free doxorubicin with respect to cardiotoxicity and mitochondrial damage. Int J Cancer. 2007 Feb 15;120(4):927-34. PubMed PMID: 17131338.
16: Di Stefano G, Lanza M, Kratz F, Merina L, Fiume L. A novel method for coupling doxorubicin to lactosaminated human albumin by an acid sensitive hydrazone bond: synthesis, characterization and preliminary biological properties of the conjugate. Eur J Pharm Sci. 2004 Dec;23(4-5):393-7. PubMed PMID: 15567293.
| EP0169111A1 * | Jun 18, 1985 | Jan 22, 1986 | Sanofi | Cytotoxic conjugates useful in therapy, and process for obtaining them |
| EP0269188A2 * | Jun 18, 1985 | Jun 1, 1988 | Elf Sanofi | Cytotoxic conjugates useful in therapy, and process for obtaining them |
| EP0306943A2 * | Sep 8, 1988 | Mar 15, 1989 | Neorx Corporation | Immunconjugates joined by thioether bonds having reduced toxicity and improved selectivity |
| EP0328147A2 * | Feb 10, 1989 | Aug 16, 1989 | Bristol-Myers Squibb Company | Anthracycline immunoconjugates having a novel linker and methods for their production |
| EP0398305A2 * | May 16, 1990 | Nov 22, 1990 | Bristol-Myers Squibb Company | Anthracycline conjugates having a novel linker and methods for their production |
| EP0457250A2 * | May 13, 1991 | Nov 21, 1991 | Bristol-Myers Squibb Company | Novel bifunctional linking compounds, conjugates and methods for their production |
KEY words
Aldoxorubicin, CytRx, CANCER, INNO-206, PHASE 3, oncology, Soft Tissue Sarcoma

























Sorry, the comment form is closed at this time.