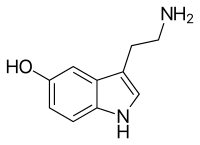

Serotonin /ˌsɛrəˈtoʊnɨn/ or 5-hydroxytryptamine (5-HT) is a monoamine neurotransmitter. Biochemically derived from tryptophan, serotonin is primarily found in the gastrointestinal (GI) tract, platelets, and the central nervous system (CNS) of animals, including humans. It is popularly thought to be a contributor to feelings of well-being and happiness.[6]
In 1935, Italian Vittorio Erspamer showed an extract from enterochromaffin cells made intestines contract. Some believed it contained adrenaline, but two years later, Erspamer was able to show it was a previously unknown amine, which he named “enteramine”. In 1948, Maurice M. Rapport, Arda Green, and Irvine Page of the Cleveland Clinic discovered a vasoconstrictor substance inblood serum, and since it was a serum agent affecting vascular tone, they named it serotonin.
In 1952, enteramine was shown to be the same substance as serotonin, and as the broad range of physiological roles was elucidated, the abbreviation 5-HT of the proper chemical name 5-hydroxytryptamine became the preferred name in the pharmacological field. Synonyms of serotonin include: 5-hydroxytriptamine, thrombotin, enteramin, substance DS, and 3-(β-Aminoethyl)-5-hydroxyindole.In 1953, Betty Twarog and Page discovered serotonin in the central nervous system.

Approximately 90% of the human body’s total serotonin is located in the enterochromaffin cells in the alimentary canal (gut), where it is used to regulate intestinal movements.[7][8] The remainder is synthesized in serotonergic neurons of the CNS, where it has various functions. These include the regulation of mood, appetite, and sleep. Serotonin also has some cognitive functions, including memory and learning. Modulation of serotonin at synapses is thought to be a major action of several classes of pharmacological antidepressants.
Serotonin secreted from the enterochromaffin cells eventually finds its way out of tissues into the blood. There, it is actively taken up by bloodplatelets, which store it. When the platelets bind to a clot, they release serotonin, where it serves as a vasoconstrictor and helps to regulatehemostasis and blood clotting. Serotonin also is a growth factor for some types of cells, which may give it a role in wound healing.

Serotonin is metabolized mainly to 5-HIAA, chiefly by the liver. Metabolism involves first oxidation by monoamine oxidase to the correspondingaldehyde. This is followed by oxidation by aldehyde dehydrogenase to 5-HIAA, the indole acetic acid derivative. The latter is then excreted by the kidneys. One type of tumor, called carcinoid, sometimes secretes large amounts of serotonin into the blood, which causes various forms of thecarcinoid syndrome of flushing (serotonin itself does not cause flushing. Potential causes of flushing in carcinoid syndrome include bradykinins, prostaglandins, tachykinins, substance P, and/or histamine.), diarrhea, and heart problems. Because of serotonin’s growth-promoting effect on cardiac myocytes,[9] persons with serotonin-secreting carcinoid may suffer a right heart (tricuspid) valve disease syndrome, caused by proliferation of myocytes onto the valve.
In addition to animals, serotonin is found in fungi and plants.[10] Serotonin’s presence in insect venoms and plant spines serves to cause pain, which is a side-effect of serotonin injection. Serotonin is produced by pathogenic amoebae, and its effect on the gut causes diarrhea. Its widespread presence in many seeds and fruits may serve to stimulate the digestive tract into expelling the seeds.
Serotonin system, contrasted with thedopamine system
|
Introduction Serotonin was first recognised as a powerful vasoconstrictor in blood serum. It was isolated in 1948 by Page and was later found to be associated with the central nervous system.
Serotonin is naturally produced in the Pineal gland which lies deep at the centre of the human brain. The average adult human possesses only 5 to 10 mg of serotonin, 90 % of which is in the intestine and the rest in blood platelets and the brain. One role of this ‘wonder drug’ is as a neurotransmitter, allowing numerous functions in the human body including the control of appetite, sleep, memory and learning, temperature regulation, mood, behaviour, cardiovascular function, muscle contraction, endocrine regulation and depression. Subsequent to his discovery of Serotonin, Page commented that no physiological substance known possesses such diverse actions in the body as does serotonin. 5-HT is also found in wasp stings and scorpion venom where its function is of an irritant, since intravenous injection of serotonin in humans leads to pain, gasping, coughing, a tingling and prickling sensation, nausea, cramps and other unpleasant symptoms. |
Serotonin is manufactured in the human brain using the essential amino acid tryptophan which is found in foods such as bananas, pineapples, plums, turkey and milk.
The enzyme tryptophan hydroxylase adds a hydroxyl group to tryptophan’s benzene ring at position 5, creating 5-hydroxytryptophan. Another enzyme, amino acid decarboxylase, then removes a carboxyl group from 5-hydroxytryptophan, forming 5-hydroxytryptamine which is more commonly known as serotonin.

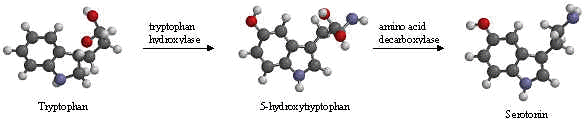

In animals including humans, serotonin is synthesized from the amino acid L–tryptophan by a short metabolic pathway consisting of two enzymes: tryptophan hydroxylase (TPH) and amino acid decarboxylase (DDC). The TPH-mediated reaction is the rate-limiting step in the pathway. TPH has been shown to exist in two forms: TPH1, found in several tissues, and TPH2, which is a neuron-specific isoform.
Serotonin can be synthesized from tryptophan in the lab using Aspergillus niger and Psilocybe coprophila as catalysts. The first phase to 5-hydroxytryptophan would require letting tryptophan sit in ethanol and water for 7 days, then mixing in enough HCl (or other acid) to bring the pH to 3, and then adding NaOH to make a pH of 13 for 1 hour. Asperigillus niger would be the catalyst for this first phase. The second phase to synthesizing tryptophan itself from the 5-hydroxytryptophan intermediate would require adding ethanol and water, and letting sit for 30 days this time. The next two steps would be the same as the first phase: adding HCl to make the pH = 3, and then adding NaOH to make the pH very basic at 13 for 1 hour. This phase uses the Psilocybe coprophila as the catalyst for the reaction.
Serotonin taken orally does not pass into the serotonergic pathways of the central nervous system, because it does not cross theblood–brain barrier. However, tryptophan and its metabolite 5-hydroxytryptophan (5-HTP), from which serotonin is synthesized, can and do cross the blood–brain barrier. These agents are available as dietary supplements, and may be effective serotonergic agents. One product of serotonin breakdown is 5-hydroxyindoleacetic acid (5-HIAA), which is excreted in the urine. Serotonin and 5-HIAA are sometimes produced in excess amounts by certain tumors or cancers, and levels of these substances may be measured in the urine to test for these tumors.
Serotonin is a neurotransmitter involved in the transmission of nerve impulses. Neurotransmitters are chemical messengers within the brain that allow the communication between nerve cells.
Packets of serotonin (vesicles) are released from the end of the presynaptic cell 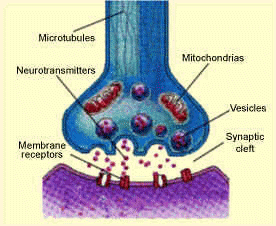 into the synaptic cleft. The serotonin molecules can then bind to receptor proteins within the postsynaptic cell, which causes a change in the electrical state of the cell. This change in electrical state can either excite the cell, passing along the chemical message, or inhibit it. Excess serotonin molecules are taken back up by the presynaptic cell and reprocessed.
into the synaptic cleft. The serotonin molecules can then bind to receptor proteins within the postsynaptic cell, which causes a change in the electrical state of the cell. This change in electrical state can either excite the cell, passing along the chemical message, or inhibit it. Excess serotonin molecules are taken back up by the presynaptic cell and reprocessed.
www.thebrain.mcgill.ca/flash/i/i_01/i_01_m/i_01_m_ana/i_01_m_ana.html
The neurons in the brain that release serotonin are found in small dense collections of neurons called Raphe Nuclei. The Raphe Nuclei are found in the medulla, pons and midbrain which are all located at the top of the spinal cord. Serotonergic neurons have axons which project to many different parts of the brain, therefore serotonin affects many different behaviors.
Low serotonin levels are believed to be the cause of many cases of mild to severe depression which can lead to symptoms such as anxiety, apathy, fear, feelings of worthlessness, insomnia and fatigue. The most concrete evidence for the connection between serotonin and depression is the decreased concentrations of serotonin metabolites in the cerebrospinal fluid and brain tissues of depressed people.
worthlessness, insomnia and fatigue. The most concrete evidence for the connection between serotonin and depression is the decreased concentrations of serotonin metabolites in the cerebrospinal fluid and brain tissues of depressed people.
http://www.depression.org/
If depression arises as a result of a serotonin deficiency then pharmaceutical agents that increase the amount of serotonin in the brain should be helpful in treating depressed patients. Anti-depressant medications increase serotonin levels at the synapse by blocking the reuptake of serotonin into the presynaptic cell. Anti-depressants are one of the most highly prescribed medications despite the serious side-effects they can cause.
If depression is mild enough it can sometimes be managed without prescribed medications. The most effective way of raising serotonin levels is with vigorous exercise. Studies have shown that serotonin levels are increased with increased activity and the production of serotonin is increased for some days after the activity. This is the safest way of increasing serotonin levels and many other benefits result from regular exercise.
Serotonin levels can also be controlled through the diet. A diet deficient in omega-3 fatty acids may lower brain levels of serotonin and cause depression. Complex carbohydrates raise the level of tryptophan in the brain resulting in a calming effect. Vitamin C is also required for the conversion of tryptophan into serotonin.
Lysergic acid diethylamide, more commonly known as LSD, is a non-toxic, non-addictive molecule which mimics serotonin in the brain. The body ‘mistakes’ LSD for serotonin and shoots it across the synaptic cleft. LSD has a higher affinity for 5-HT receptors than serotonin, thus the presence of LSD prevents
 serotonin from sending neural messages in the brain. Once the LSD molecule is bound to the receptor proteins the message is not carried any further. Instead the impulse is redirected to the older parts of the brain, where the bloodstream then takes it to the sense interpretive centres and the motor areas.
serotonin from sending neural messages in the brain. Once the LSD molecule is bound to the receptor proteins the message is not carried any further. Instead the impulse is redirected to the older parts of the brain, where the bloodstream then takes it to the sense interpretive centres and the motor areas.
There are many similarities between the molecules of serotonin and LSD which allows this process to occur, the most obvious being their close structural similarities, particularly the indole ring shown highlighted in blue.
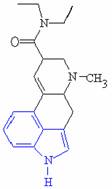
Serotonin Lysergic acid diethylamide
Another close similarity between LSD and serotonin is the electron density of the highest occupied molecular orbital. The electron density is lowest in the areas around the indole ring in both molecules. This is indicated by the blue areas in the diagrams.
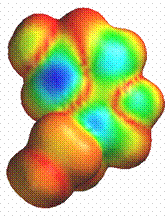
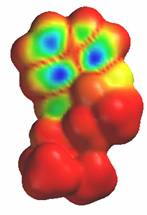
Serotonin LSD
The dipole moment of the two molecules are very close. Serotonin is 2.98 debye and LSD is 3.04 debye, with the dipole moment going towards the NH2 group in both molecules. The close similarity in dipole moment is key to the ability of LSD to fit into the same receptors as serotonin.
The combination of all of these chemical similarities allows LSD to imitate serotonin and cause psychedelic hallucinations and visions.
|
- Pietra, S.;Farmaco, Edizione Scientifica 1958, Vol. 13, pp. 75–9.
- Calculated using Advanced Chemistry Development (ACD/Labs) Software V11.02 (©1994–2011 ACD/Labs)
- Mazák, K.; Dóczy, V.; Kökösi, J.; Noszál, B. (2009). “Proton Speciation and Microspeciation of Serotonin and 5-Hydroxytryptophan”. Chemistry & Biodiversity 6 (4): 578–90.doi:10.1002/cbdv.200800087. PMID 19353542.
- Erspamer, Vittorio (1952). Ricerca Scientifica 22: 694–702.
- Tammisto, Tapani (1968). Annales Medicinae Experimentalis et Biologiea Fenniae 46 (3, Pt. 2): 382–4.
- Young SN (2007). “How to increase serotonin in the human brain without drugs”. Rev. Psychiatr. Neurosci. 32(6): 394–99. PMC 2077351. PMID 18043762.
- King MW. “Serotonin”. The Medical Biochemistry Page. Indiana University School of Medicine. Retrieved 1 December 2009.
- Berger M, Gray JA, Roth BL (2009). “The expanded biology of serotonin”. Annu. Rev. Med. 60: 355–66.doi:10.1146/annurev.med.60.042307.110802.PMID 19630576.
- Bianchi, P. (2005). “A new hypertrophic mechanism of serotonin in cardiac myocytes: Receptor-independent ROS generation”. The FASEB Journal. doi:10.1096/fj.04-2518fje.
- Kang K, Park S, Kim YS, Lee S, Back K (2009). “Biosynthesis and biotechnological production of serotonin derivatives”. Appl. Microbiol. Biotechnol. 83 (1): 27–34.doi:10.1007/s00253-009-1956-1. PMID 19308403.

| www.encyclopedia.com/html/s1/serotoni.asp |
| www.angelfire.com/hi/TheSeer/seratonin.html |
| www.findthelight.net/Depression/the_chemistry_of_dep.htm |
| http://www.cmste.uncc.edu/Document%20Hold/Sawsun-%20Serotonin%20FINAL%20PAPER.doc |
| www.totse.com/en/technology/science_technology/seroton.html |
| www.macalester.edu/~psych/whathap/ubnrp/mdma/serotonin.html |
| www.serendipity.li/mcclay/pineal.html#a1.6 |
| www.serendip.brynmawr.edu/bb/neuro/neuro98/202s98-paper3/Frederickson3.html |
| www.serendip.brynmawr.edu/bb/neuro/neuro99/web1/Byrd.html |













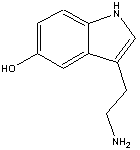 The chemical name for serotonin is 5-hydoxytryptamine which is often abbreviated to 5-HT.
The chemical name for serotonin is 5-hydoxytryptamine which is often abbreviated to 5-HT.
Sorry, the comment form is closed at this time.