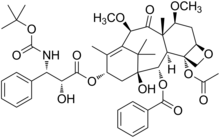
Cabazitaxel
For treatment of patients with hormone-refractory metastatic prostate cancer previously treated with a docetaxel-containing treatment regimen.
4-acetoxy-2α-benzoyloxy-5β,20-epoxy-1-hydroxy-7β,10β-dimethoxy-9-oxotax-11-en-13α-yl(2R,3S)-3-tert-butoxycarbonylamino-2-hydroxy-3-phenyl-propionate
(1S,2S,3R,4S,7R,9S,10S,12R,15S)-4-(Acetyloxy)-15-{[(2R,3S)-3-{[(tert-butoxy)carbonyl]amino}-2-hydroxy-3-phenylpropanoyl]oxy}-1-hydroxy-9,12-dimethoxy-10,14,17,17-tetramethyl-11-oxo-6-oxatetracyclo[11.3.1.03,10.04,7]heptadec-13-ene-2-yl benzoate
183133-96-2
Cabazitaxel is prepared by semi-synthesis from 10-deacetylbaccatin III (10-DAB) which is extracted from yew tree needles. The chemical name of cabazitaxel is (2α,5β,7β,10β,13α)-4-acetoxy-13-({(2R,3S)-3-[(tert-butoxycarbonyl)amino]-2-hydroxy-3-phenylpropanoyl}oxy)-1-hydroxy-7,10-dimethoxy-9-oxo-5,20-epoxy-tax-11-en-2-yl benzoate and is marketed as a 1:1 acetone solvate (propan-2-one),
Cabazitaxel is an anti-neoplastic used with the steroid medicine prednisone. Cabazitaxel is used to treat people with prostate cancer that has progressed despite treatment with docetaxel. Cabazitaxel is prepared by semi-synthesis with a precursor extracted from yew needles (10-deacetylbaccatin III). It was approved by the U.S. Food and Drug Administration (FDA) on June 17, 2010.
Cabazitaxel (previously XRP-6258, trade name Jevtana) is a semi-synthetic derivative of a natural taxoid.[1] It was developed by Sanofi-Aventis and was approved by the U.S. Food and Drug Administration (FDA) for the treatment of hormone-refractory prostate cancer on June 17, 2010. It is a microtubule inhibitor, and the fourth taxane to be approved as a cancer therapy.[2]
Nagesh Palepu, “CABAZITAXEL FORMULATIONS AND METHODS OF PREPARING THEREOF.” U.S. Patent US20120065255, issued March 15, 2012.
Cabazitaxel in combination with prednisone is a treatment option for hormone-refractory prostate cancer following docetaxel-based treatment.
Clinical trials
In a phase III trial with 755 men for the treatment of castration-resistant prostate cancer, median survival was 15.1 months for patients receiving cabazitaxel versus 12.7 months for patients receiving mitoxantrone. Cabazitaxel was associated with more grade 3–4 neutropenia (81.7%) than mitoxantrone (58%).[3]
| United States | 5438072 | 2010-06-17 | exp 2013-11-22 |
| United States | 5698582 | 2010-06-17 | 2012-07-03 |
| United States | 5847170 | 2010-06-17 | 2016-03-26 |
| United States | 6331635 | 2010-06-17 | 2016-03-26 |
| United States | 6372780 | 2010-06-17 | 2016-03-26 |
| United States | 6387946 | 2010-06-17 | 2016-03-26 |
| United States | 7241907 | 2010-06-17 | 2025-12-10 |
JEVTANA (cabazitaxel) is an antineoplastic agent belonging to the taxane class. It is prepared by semi-synthesis with a precursor extracted from yew needles.
The chemical name of cabazitaxel is (2α,5β,7β,10β,13α)-4-acetoxy-13-({(2R,3S)-3[(tertbutoxycarbonyl) amino]-2-hydroxy-3-phenylpropanoyl}oxy)-1-hydroxy-7,10-dimethoxy-9oxo-5,20-epoxytax-11-en-2-yl benzoate – propan-2-one(1:1).
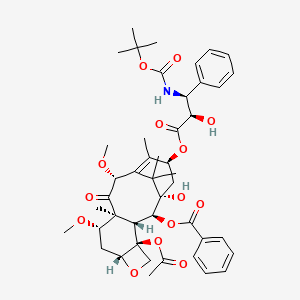
Cabazitaxel has the following structural formula:
 |
Cabazitaxel is a white to almost-white powder with a molecular formula of C45H57NO14•C3H6O and a molecular weight of 894.01 (for the acetone solvate) / 835.93 (for the solvent free). It is lipophilic, practically insoluble in water and soluble in alcohol.
JEVTANA (cabazitaxel) Injection 60 mg/1.5 mL is a sterile, non-pyrogenic, clear yellow to brownish-yellow viscous solution and is available in single-use vials containing 60 mg cabazitaxel (anhydrous and solvent free) and 1.56 g polysorbate 80. Each mL contains 40 mg cabazitaxel (anhydrous) and 1.04 g polysorbate 80.
DILUENT for JEVTANA is a clear, colorless, sterile, and non-pyrogenic solution containing 13% (w/w) ethanol in water for injection, approximately 5.7 mL.
JEVTANA requires two dilutions prior to intravenous infusion. JEVTANA injection should be diluted only with the supplied DILUENT for JEVTANA, followed by dilution in either 0.9% sodium chloride solution or 5% dextrose solution.
The taxane family of terpenes has received much attention in the scientific and medical community, because members of this family have demonstrated broad spectrum of anti-leukemic and tumor-inhibitory activity. A well-known member of this family is paclitaxel (Taxol®).
Paclitaxel (Taxol) Paclitaxel was first isolated from the bark of the pacific yew tree (Taxus brevifolia) in 1971 , and has proved to be a potent natural anti-cancer agent. To date, paclitaxel has been found to have activity against different forms of leukemia and against solid tumors in the breast, ovary, brain, and lung in humans.
As will be appreciated, this beneficial activity has stimulated an intense research effort over recent years with a view to identifying other taxanes having similar or improved properties, and with a view to developing synthetic pathways for making these taxanes, such as paclitaxel.
This research effort led to the discovery of a synthetic analogue of paclitaxel, namely, docetaxel (also known as Taxotere®). As disclosed in U.S. Patent No. 4,814,470, docetaxel has been found to have a very good anti-tumour activity and better bioavailability than paclitaxel. Docetaxel is similar in structure to paclitaxel, having t- butoxycarbonyl instead of benzoyl on the amino group at the 3′ position, and a hydroxy group instead of the acetoxy group at the C-10 position.
As will be appreciated, taxanes are structurally complicated molecules, and the development of commercially viable synthetic methods to make taxanes has been a challenge. A number of semi-synthetic pathways have been developed over the years, which typically begin with the isolation and purification of a naturally occurring starting material, which can be converted to a specific taxane derivative of interest. Cabazitaxel (I) is an anti-tumor drug which belongs to the taxol family. It differs from docetaxel in that it has methoxy groups at positions 7 and 10 of the molecule, as opposed to the hydroxyl groups at equivalent positions in docetaxel. Cabazitaxel is obtained by semi-synthesis from an extract of Chinese yew (Taxus mairei). It is understood that cabazitaxel can be obtained via semi-synthesis from other taxus species including T.candensis, T.baccatta, T.chinensis, T. mairei etc.
Cabazitaxel is a semi-synthetic derivative of the natural taxoid 0-deacetylbaccatin III (10-DAB) with potentially unique antineoplastic activity for a variety of tumors.
Cabazitaxel binds to and stabilizes tubulin, resulting in the inhibition of microtubule depolymerization and cell division, cell cycle arrest in the G2/M phase, and the inhibition of tumor cell proliferation. This drug is a microtubule depolymerization inhibitor, which can penetrate blood brain barrier (BBB).
Cabazitaxel was recently approved by the US Federal Drug Administration (FDA) for the treatment of docetaxel resistant hormone refractory prostate cancer. It has been developed by Sanofi-Aventis under the trade name of Jevtana. The CAS number for the compound is 183133-96-2. A synonym is dimethoxydocetaxel. The compound is also known as RPR-1 16258A; XRP6258; TXD 258; and axoid XRP6258.
The free base form of cabazitaxel has the chemical name
(2aR,4S,4aS,6R,9S, 1 1 S,12S,12aR, 12bS)-12b-acetoxy-9-(((2R,3S)-3-((tert- butoxycarbonyl)amino)-2-hydroxy-3-phenylpropanoyl)oxy)-11-hydroxy-4,6-dimethoxy- 4a,8, 13, 13-tetramethyl-5-oxo-2a,3,4,4a,5,6,9, 10, 11 , 12, 12a, 12b-dodecahydro-1 H- 7, 1 1-methanocyclodeca[3,4]benzo[1 ,2-b]oxet-12-yl benzoate. In a first part of this description, taxel drugs including paclitaxel (taxol), docetaxel (taxotere) and cabazitaxel may be prepared starting from 10-deacetylbaccatin (known as 10-DAB) derived from Taxus plants, via semi-synthesis. Furthermore, the same inventive methodologies can be used to semi-synthesize cabazitaxel starting from 9- dihydro-13-acetylbaccatin III (9-DHB).
Patent numbers CN1213042C, CN152870, CN1179716 and CN1179775 disclose methods to prepare cabazitaxel from 10-DAB (herein compound II).
10-DAB (II)
A typical prior art synthesis route is as follows:
OCOCH3
OCOC6H5
The method above which synthesizes cabazitaxel has many synthetic steps, a very low overall yield and high price.
There is therefore a need in the art to develop new methods to synthesize cabazitaxel and its intermediates to improve the yield of cabazitaxel, simplify the methodology and optimize the synthetic technology.
Cabazitaxel, chemically known as 4-acetoxy-2α-benzoyloxy-5β,20-epoxy-1-hydroxy-7β,10β-dimethoxy-9-oxotax-11-en-13α-yl(2R,3S)-3-tert-butoxycarbonylamino-2-hydroxy-3-phenyl-propionate, is represented by formula (I).
It is a microtubule inhibitor, indicated in combination with prednisone for treatment of patients with hormone-refractory metastatic prostate cancer previously treated with a docetaxel-containing treatment regimen, under the trade name Jevtana®.
Cabazitaxel is known from U.S. Pat. No. 5,847,170. Process for preparation of Cabazitaxel as described in U.S. Pat. No. 5,847,170 involves column chromatography, which is cumbersome tedious and not commercially viable.
The acetone solvate of 4-acetoxy-2α-benzoyloxy-5β-20-epoxy-1-hydroxy-7β, 10β-dimethoxy-9-oxotan-11-en-13α-yl-(2R,3S)-3-tert-butoxycarbonylamino-2-hydroxy-3-phenylpropionate (Form A) is formed by crystallization by using acetone and is characterized by XRD in U.S. Pat. No. 7,241,907.
U.S. 20110144362 describes anhydrous crystalline Forms B to Form F, ethanolates Form B, D, E and F and mono and dihydrate Forms of Cabazitaxel. All the anhydrous crystalline forms are prepared either by acetone solvate or ethanol solvate. Mono and dihydrate forms are formed at ambient temperature in an atmosphere containing 10 and 60% relative humidity, respectively.
Cabazitaxel (also called dimethoxy docetaxel) is a dimethyl derivative of docetaxel, which itself is semi-synthetic, and was originally developed by Rhone-Poulenc Rorer and was approved by the U.S. Food and Drug Administration (FDA) for the treatment of hormone-refractory prostate cancer on Jun. 17, 2010. Cabazitaxel is a microtubule inhibitor. The acetone solvate crystalline form of cabazitaxel and a process for its preparation is disclosed in the U.S. Pat. No. 7,241,907.
U.S. Pat. No. 5,847,170 describes cabazitaxel and its preparation methods. One of the methods described in U.S. Pat. No. 5,847,170 includes a step-wise methylation of 10-DAB (the step-wise methylation method is shown in FIG. 1) to provide the key intermediate (2αR,4S,4αS,6R,9S,11S,12S,12αR,12βS)-12β-acetoxy-9,11-dihydroxy-4,6-dimethoxy-4α,8,13,13-tetramethyl-5-oxo-2α,3,4,4α,5,6,9,10,11,12,12α,12β-dodecahydro-1H-7,11-methanocyclodeca[3,4]benzo[1,2-b]oxet-12-yl benzoate, herein referred to as 7,10-di-O-methyl-10-DAB (XVa). The intermediate XVa is coupled with the 3-phenylisoserine side chain derivative VI to provide XVa′, which is followed by removal of the oxazolidine protecting group from the side chain of XVa′ to give cabazitaxel.
Another method described in U.S. Pat. No. 5,847,170 utilizes methylthiomethyl (MTM) ethers as shown in FIG. 2. MTM ethers can be prepared from alcohols using two common methods. One method comprises deprotonation of an alcohol with a strong base to form an alkoxide followed by alkylation of the alkoxide with a methylthiomethyl halide. This approach is only useful when the alcohol is stable to treatment with a strong base. 10-DAB and some of its derivatives in which C7-OH is not protected displays so instability in the presence of strong bases and epimerization of the C7-OH can occur upon contact of 10-DAB and some of its derivatives in which C7-OH is not protected with strong bases. Another method for the synthesis of MTM ethers from alcohols utilizes Ac2O and DMSO. One disadvantage of this method is that it can also lead to the oxidation of alcohols to aldehydes or ketones. For example when the synthesis of the 10-di-O-MTM derivative of 10-DAB without protecting groups at the C13 hydroxyl group is attempted undesired oxidation of the C13-OH to its corresponding ketone occurs.
U.S. Pat. No. 5,962,705 discloses a method for dialkylation of 10-DAB and its derivatives to furnish 7,10-di-O-alkyl derivatives, as shown in FIG. 3. This has been demonstrated as a one-step, one-pot reaction, however, provides the best isolated yield when potassium hydride is used at −30° C. From an industrial point of view, the use of low reaction temperature is less favorable than using ambient temperature. Furthermore the use of a strong base can cause some epimerization of the C7-OH chiral center with an associated loss of yield. Potassium hydride is a very reactive base and must be treated with great caution.
Accordingly, there is a need for an alternative processes for the preparation of cabazitaxel and its key intermediate, 7,10-di-O-methyl-10-DAB (XVa) that is short in number of synthetic steps and avoids the use of low temperatures and strong bases such as metal hydrides in the C7-O methyl ether formation step. Such a process would also be useful for the preparation of analogues of cabazitaxel wherein the C7-O and C10-O functional groups were substituted with other alkyl groups.
FIG. 1 shows the chemistry employed in the examples of U.S. Pat. No. 5,847,170.
FIG. 2 shows the chemistry employed in the examples of U.S. Pat. No. 5,847,170.
FIG. 3 shows the chemistry employed in the examples of U.S. Pat. No. 5,962,705.
FIG. 4 shows key steps of the general synthetic scheme as per Method A/A′ of the present invention for the synthesis of cabazitaxel and cabazitaxel analogues.
FIG. 5 shows key steps of the general synthetic scheme as per Method B/B′ of the present invention for the synthesis of cabazitaxel and cabazitaxel analogues.
FIG. 6 shows the general scheme for the hydrodesulfurization reaction.
FIG. 7 shows the complete synthetic route of Method A that can be used for conversion of 10-DAB to cabazitaxel.
FIG. 8 shows the complete synthetic route of Method B that can be used for conversion of 10-DAB to cabazitaxel.
FIG. 9 shows the complete synthetic route of Method A′ that can be used for conversion of 10-DAB, via XIV′, to cabazitaxel.
FIG. 10 shows the complete synthetic route of Method B′ that can be used for conversion of 10-DAB, via XVI′, to cabazitaxel.
FIG. 11 shows the synthetic relationship between two methods (A and B) used to convert 7,10-di-O-alkyl-10-DAB (XV) to cabazitaxel.
FIG. 12 shows the synthetic scheme for the preparation of XIVa.
FIG. 13 shows the synthetic scheme for the preparation of XIVb from 10-DAB.
FIG. 14 shows the synthetic scheme for the preparation of XIVb from XIVb′.
FIG. 15 shows the synthetic scheme for the preparation of XIVc.
FIG. 16 shows the synthetic scheme for the preparation of XIVa′ from XIVa.
FIG. 17 shows the synthetic scheme for the preparation of XIVa′ from XX.
FIG. 18 shows the synthetic scheme for the preparation of XIVb′.
FIG. 19 shows the synthetic scheme for the preparation of XIVc′.
FIG. 20 shows the synthetic scheme for the preparation of XVa from XIVa.
FIG. 21 shows the synthetic scheme for the preparation of XVa from XIVb.
FIG. 22 shows the synthetic scheme for the preparation of XVa from XIVc.
FIG. 23 shows the synthetic scheme for the preparation of XVa′ from XIVa.
FIG. 24 shows the synthetic scheme for the preparation of XVa′ from XIVa′.
FIG. 25 shows the synthetic scheme for the preparation of XVa′ from XIVb′.
FIG. 26 shows the synthetic scheme for the preparation of XVa′ from XIVc′.
FIG. 27 shows the synthetic scheme for the preparation of XVa′ from XVa.
FIG. 28 shows the synthesis of cabazitaxel.
FIG. 29 shows the synthesis of XVIIa.
FIG. 30 shows the synthesis of XVIIIa.
FIG. 31 shows the synthesis of XIXa.
FIG. 32 shows the synthesis of XVIa.
FIG. 33 shows the synthesis of XVa from XVIa.











see this at http://www.google.com/patents/US20130116444
………..
Detailed description
The invention provides a new method for the preparation of cabazitaxel, one embodiment of which can be summarized as follows, showing the preparation of a protected taxane intermediate and its deprotection to taxane compounds:
OH OCOCH3
OCOC6H5
This reaction is also depicted in Figure 1. The reaction of the invention reduces the number of steps and increases yield of cabazitaxel.
The deprotection methods of the invention can also be used for the preparation of paclitaxel (taxol):
The deprotection methods of the invention can also be applied to the preparation of docetaxel:
10-DAB synthetic routes
Example 12
Dissolve 100 g of 2′-THP-cabazitaxel in 1730 ml of HOAc/H20/THF (3:1 :1 ). under N2 atmosphere, increase temperature to 50 degrees C and stir 4 hrs. Then cool to room temperature. Add 2L of ethyl acetate, 2 L of H20, stir, separate layers, wash organic layer with saturated NaHC03 (3 L x 2), saturated NaCI (3 L), dry with Na2S04.
Concentrate to obtain white 77.8 g of cabazitaxel (yield 83%).
MS(m/z) :859(M+Na)„ jHNMR (500MHz) δ 1.21(611, d) , 1.36(911, s) , 1.59(lH, s) , 1.64(lH,s) , 1.79(lH,m) , 1.87 (3H, s) ,2.27 (2H, m) , 2.35(3H,m) ,2.69(lH,m) ,3.30 (3H, s) ,
3.45 (3H, s) , 3.85 (2H, m) , 4.16 (1H, d) , 4.29 (1H, d) , 4.62 (1H, bs) , 4.79 (1H, s) , 5.29 (1H, m),5.42(lH, d),5.62(lH, d),6.21 (1H, t),7.2 ~ 7.4(6H, m) , 7.48 (2H, t),7.59(lH, t) , 8.11 (2H, d) ,
References
- http://www.cancer.gov/drugdictionary/?CdrID=534131
- “Jevtana (cabazitaxel) Injection Approved by U.S. FDA After Priority Review” (Press release). sanofi-aventis. 2010-06-17. Retrieved June 17, 2010.
- “Cabazitaxel Effective for Hormone Refractory Prostate Cancer After Failure of Taxotere”.
- Cabazitaxel – Official web site of manufacturer.
- Cabazitaxel Prescribing Information – Official prescribing information.
- U.S. National Library of Medicine: Drug Information Portal – Cabazitaxel
Patents
Patent :
Patent Number : 5438072
Country : United States
Approved : 2010-06-17
Expires : 2013-11-22
Patent :
Patent Number : 5698582
Country : United States
Approved : 2010-06-17
Expires : 2012-07-03
Patent :
Patent Number : 5847170
Country : United States
Approved : 2010-06-17
Expires : 2016-03-26
Patent :
Patent Number : 6331635
Country : United States
Approved : 2010-06-17
Expires : 2016-03-26
Patent :
Patent Number : 6372780
Country : United States
Approved : 2010-06-17
Expires : 2016-03-26
Patent :
Patent Number : 6387946
Country : United States
Approved : 2010-06-17
Expires : 2016-03-26
Patent :
Patent Number : 7241907
Country : United States
Approved : 2010-06-17
Expires : 2025-12-10
| US2009069411 | 3-13-2009 | SELF-EMULSIFYING AND SELF-MICROEMULSIFYING FORMULATIONS FOR THE ORAL ADMINISTRATION OF TAXOIDS |
| US2005070496 | 3-32-2005 | Semi-solid formulations for the oral administration of taxoids |
| US2005025792 | 2-4-2005 | Self-emulsifying and self-microemulsifying formulations for the oral administration of taxoids |
| US6403634 | 6-12-2002 | Use of taxoid derivatives |


























Sorry, the comment form is closed at this time.