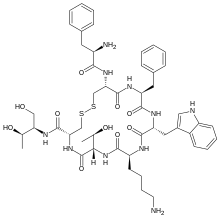
Octreotide
(D)-Phe-Cys-Phe-(D)-Trp-Lys-Thr-Cys-Thr-ol.
(4R,7S,10S,13R,16S,19R)-10-(4-aminobutyl)-19-[[(2R)-2-amino-3-phenyl-propanoyl]amino]-16-benzyl-N-[(2R,3R)-1,3-dihydroxybutan-2-yl]-7-(1-hydroxyethyl)-13-(1H-indol-3-ylmethyl)-6,9,12,15,18-pentaoxo-1,2-dithia-5,8,11,14,17-
pentazacycloicosane-4-carboxamide
L-cysteinamide, D-phenylalanyl-L-cysteiny-L-phenylalanyl-D-tryptophyl-L-lysyl-L-threonyl-N-[2-hydroxy-1-(hydroxymethyl)propyl]-,cyclic (2→7)-disulfide; [R-(R*,R*)].
Octreotide is the acetate salt of a cyclic octapeptide. It is a long-acting octapeptide with pharmacologic properties mimicking those of the natural hormone somatostatin.
| Canada | 1328402 | 1994-04-12 | expiry 2011-04-12 |
| United States | 5922338 | 1997-01-13 | 2017-01-13 |
| United States | 5538739 | 1993-07-23 | 2013-07-23 |
| CAS number | 83150-76-9 79517-01-4 (acetate) 135467-16-2 (pamoate) |
|---|
Sandostatin LAR Depot
L-Cysteinamide, D-phenylalanyl-L-cysteinyl-L-phenylalanyl-D-tryptophyl-L-lysyl-L-threonyl-N-(2-hydroxy-1-(hydroxymethyl)propyl)-, cyclic(2-7)-disulfide, (R-(R*,R*))-, acetate (salt)
Octreotide Acetate Depot
AC1L1GVR
AC1Q2BPN
CCRIS 8708
Octreotide acetate [USAN:JAN]
UNII-75R0U2568I
83150-76-9 (Parent)
AC-663
Octreotide (brand name Sandostatin,[1] Novartis Pharmaceuticals) is an octapeptide that mimics natural somatostatin pharmacologically, though it is a more potent inhibitor of growth hormone, glucagon, and insulin than the natural hormone. It was first synthesized in 1979 by the chemist Wilfried Bauer.
Since octreotide resembles somatostatin in physiological activities, it can:
- inhibit secretion of many hormones, such as gastrin, cholecystokinin, glucagon, growth hormone, insulin, secretin, pancreatic polypeptide, TSH, and vasoactive intestinal peptide,
- reduce secretion of fluids by the intestine and pancreas,
- reduce gastrointestinal motility and inhibit contraction of the gallbladder,
- inhibit the action of certain hormones from the anterior pituitary,
- cause vasoconstriction in the blood vessels, and
- reduce portal vessel pressures in bleeding varices.
It has also been shown to produce analgesic effects, most probably acting as a partial agonist at the mu opioid receptor.[2][3]
Acromegaly is a hormonal disorder that results when the pituitary gland produces excess growth hormone (GH). It most commonly affects middle-aged adults and can result in serious illness and premature death. Once recognized, acromegaly is treatable in most patients, but because of its slow and often insidious onset, it frequently is not diagnosed correctly.
Octreotide is one drug used to treat acromegaly. Octreotide exerts pharmacologic actions similar to those of the natural hormone somatostatin. Octreotide decreases GH and IGF-1 levels, as well as glucagons and insulin. Octreotide also suppresses luteinizing hormone (LH) response to gonadotropin releasing hormone (GnRH), decreases splanchnic blood flow, and inhibits the release of serotonin, gastrin, vasoactive intestinal peptide, secretin, motilin, and pancreatic polypeptide. In many patients, GH levels fall within one hour and headaches improve within minutes after the injection of octreotide. Several studies have shown that octreotide is effective for long-term treatment. Octreotide also has been used successfully to treat patients with acromegaly caused by non-pituitary tumors. In some acromegaly patients who already have diabetes, octreotide can reduce the need for insulin and improve blood sugar control.
Octreotide is currently available as Sandostatin LAR® Depot, which is, upon reconstitution, a suspension of microspheres containing octreotide acetate. Sandostatin LAR® Depot is the only medication indicated for the long-term maintenance therapy in acromegalic patients. It is also indicated for the long-term treatment of severe diarrhea and flushing episodes associated with metastatic carcinoid tumors and profuse water diarrhea associated with VIP-secreting tumors. Sandostatin LAR® T Depot is administered via intramuscular injection every four weeks, following a titration period. Octreotide acetate has also been available in an immediate-release formulation, Sandostatin® Injection solution, which was required to be administered by injection three times daily.
Octreotide is an octapeptide with the following amino acid sequence: L-cysteinamide, D-phenylalanyl-L-cysteiny-L-phenylalanyl-D-tryptophyl-L-lysyl-L-threonyl-N-[2-hydroxy-1-(hydroxymethyl)propyl]-,cyclic (2→7)-disulfide; [R-(R*,R*)]. The structure of octreotide is shown below.
The chemical formula is C49H66N10O10S2 and its molecular weight is 1019.3 Da. Its therapeutic category is gastric antisecretory agent.
The Food and Drug Administration (FDA) has approved the usage of a salt form of this peptide, octreotide acetate, as an injectable depot formulation for the treatment of growth hormone producing tumors (acromegaly and gigantism), pituitary tumors that secrete thyroid stimulating hormone(thyrotropinoma), diarrhea and flushing episodes associated with carcinoid syndrome, and diarrhea in patients with vasoactive intestinal peptide-secreting tumors (VIPomas).
Octreotide is used in nuclear medicine imaging by labelling with indium-111 (Octreoscan) to noninvasively image neuroendocrine and other tumours expressing somatostatin receptors.[4] More recently, it has been radiolabelled with carbon-11[5] as well as gallium-68, enabling imaging with positron emission tomography (PET), which provides higher resolution and sensitivity.
Octreotide can also be labelled with a variety of radionuclides, such as yttrium-90 or lutetium-177, to enable peptide receptor radionuclide therapy(PRRT) for the treatment of unresectable neuroendocrine tumours.
Octreotide is the acetate salt of a cyclic octapeptide. It is a long-acting octapeptide with pharmacologic properties mimicking those of the natural hormone somatostatin. Octreotide is known chemically as L-Cysteinamide, D-phenylalanyl-L-cysteinyl-L-phenylalanyl-D-tryptophyl-L-lysyl-L-threonyl-N-[2-hydroxy-1- (hydroxy-methyl) propyl]-, cyclic (2→7)-disulfide; [R-(R*,R*)].
Sandostatin LAR Depot is available in a vial containing the sterile drug product, which when mixed with diluent, becomes a suspension that is given as a monthly intragluteal injection. The octreotide is uniformly distributed within the microspheres which are made of a biodegradable glucose star polymer, D,L-lactic and glycolic acids copolymer. Sterile mannitol is added to the microspheres to improve suspendability.
Sandostatin LAR Depot is available as: sterile 5-mL vials in 3 strengths delivering 10 mg, 20 mg, or 30 mg octreotide-free peptide. Each vial of Sandostatin LAR Depot delivers:
| NAME OF INGREDIENT | 10 MG | 20 MG | 30 MG |
| octreotide acetate | 11.2 mg* | 22.4 mg* | 33.6 mg* |
| D, L-lactic and glycolic acids copolymer | 188.8 mg | 377.6 mg | 566.4 mg |
| mannitol | 41.0 mg | 81.9 mg | 122.9 mg |
| *Equivalent to 10/20/30 mg octreotide base. | |||
Each syringe of diluent contains:
| carboxymethylcellulose sodium | 12.5 mg |
| mannitol | 15.0 mg |
| water for injection | 2.5 mL |
The molecular weight of octreotide is 1019.3 (free peptide, C49H66N10O10S2) and its amino acid sequence is
 |
Octreotide has also been used off-label for the treatment of severe, refractory diarrhea from other causes. It is used in toxicology for the treatment of prolonged recurrent hypoglycemia after sulfonylurea and possibly meglitinides overdose. It has also been used with varying degrees of success in infants with nesidioblastosis to help decrease insulin hypersecretion.
Octreotide has been used experimentally to treat obesity, particularly obesity caused by lesions in the hunger and satiety centers of thehypothalamus, a region of the brain central to the regulation of food intake and energy expenditure.[6] The circuit begins with an area of the hypothalamus, the arcuate nucleus, that has outputs to the lateral hypothalamus (LH) and ventromedial hypothalamus (VMH), the brain’s feeding and satiety centers, respectively.[7][8] The VMH is sometimes injured by ongoing treatment for acute lymphoblastic leukemia (ALL) or surgery or radiation to treat posterior cranial fossa tumors.[6] With the VMH disabled and no longer responding to peripheral energy balance signals,
Octreotide has also been investigated for patients with pain from chronic pancreatitis,[11] and it may be useful in the treatment of thymic neoplasms.
The drug has been used off-label, injected subcutaneously, in the management of hypertrophic pulmonary osteoarthropathy (HPOA) secondary to non-small cell lung carcinoma. Although its mechanism is not known, it appears to reduce the pain associated with HPOA.[citation needed]
It has been used in the treatment of malignant bowel obstruction.[12]
Octreotide may be used in conjunction with midodrine to partially reverse peripheral vasodilation in the hepatorenal syndrome. By increasing systemic vascular resistance, these drugs reduce shunting and improve renal perfusion, prolonging survival until definitive treatment with liver transplant.[13] Similarly, octreotide can be used to treat refractory chronic hypotension.[14]
While successful treatment has been demonstrated in case reports,[15][16] larger studies have failed to demonstrate efficacy in treating chylothorax.[17]
Octreotide is often give as an infusion for management of acute haemorrhage from esophageal varices in liver cirrhosis on the basis that it reduces portal venous pressure, though current evidence suggests that this effect is transient and does not improve survival.[18]
A small study has shown that octreotide may be effective in the treatment of idiopathic intracranial hypertension.[19][20]
Octreotide has not been adequately studied for the treatment of children, pregnant and lactating women. The drug is given to these groups of patients only if a risk-benefit analysis is positive.[21][22]
 Acetate
Acetate
C53H74N10O14S2 , 1139.34326
The most frequent adverse effects (more than 10% of patients) are headache, hypothyroidism, cardiac conduction changes, gastrointestinal reactions (including cramps, nausea/vomiting and diarrhoea or constipation), gallstones, reduction of insulin release, hyperglycemia[23] or hypoglycemia, and (usually transient) injection site reactions. Slow heart rate, skin reactions such aspruritus, hyperbilirubinemia, hypothyroidism, dizziness and dyspnoea are also fairly common (more than 1%). Rare side effects include acute anaphylactic reactions, pancreatitis andhepatitis.[21][22] One study reported a possible association with rheumatoid arthritis.[24]
Some studies reported alopecia in patients who were treated by octreotide.[25] Rats which were treated by octreotide experienced erectile dysfunction in a 1998 study.[26]
A prolonged QT interval has been observed in patients, but it is uncertain whether this is a reaction to the drug or part of the patients’ illnesses.[21]
| Octreotide can reduce the intestinal resorption of ciclosporin, possibly making it necessary to increase the dose.[27] Patients with diabetes mellitusmight need less insulin or oral antidiabetics when treated with octreotide. The bioavailability of bromocriptine is increased;[22] besides being anantiparkinsonian, bromocriptine is also used for the treatment of acromegaly. |
|---|
Octreotide is absorbed quickly and completely after subcutaneous application. Maximal plasma concentration is reached after 30 minutes. The elimination half-life is 100 minutes (1.7 hours) on average when applied subcutaneously; after intravenous injection, the substance is eliminated in two phases with half-lives of 10 and 90 minutes, respectively.[21][22]
Conventional synthesis of octreotide may be divided into two main approaches, liquid-phase synthesis and solid-phase synthesis. · Octreotide first disclosed in US4395403, in which Octreotide is prepared by solution phase peptide synthesis. The process comprises; removing protected group from peptide;‘ linking together by an amide bond to two peptide unit; converting a function group at the N- or C- terminal; oxidizing a straight chain polypeptide by boron tristrifluoroacetate.
Since all the synthesis steps are carried out in liquid phase, US’403 process is a time- consuming, multi-step synthesis and it is difficult to separate octreotide from the reaction mixtures. Another solution phase approach described in US6987167 and WO2007110765A2, in which the cyclization of partially deprotected octreotide is carried out in the solution phase using iodine under specific conditions in presence of alcoholic solvents.
US6346601 B1 , WO2005087794A1 and WO2010089757A2 disclose a process for the preparation of octreotide by hybrid approach i. e synthesis of fragments on solid phase and condensing the obtained fragments in a liquid phase.
US6476186 describes the solid phase synthesis, in which the synthesis of octreotide using Thr(ol)(tBu)-2CI-trityl resin as starting material, followed by the cleavage of the straight chain peptide from the resin using a strong acid and the formation of the intra-molecular disulfide bond on the completely deprotected octreotide by oxidation using charcoal catalyst.
US20040039161A1 provides a solid phase .peptide synthetic method for the preparation of C-terminal alcohols using trichloroacetimidate activated linker, making the required peptide chain on the resin support, cleaving the attached peptide; air oxidation to form said C- terminal amino alcohol containing peptide and a 36.3% yield of octreotide after HPLC purification.
Charcoal oxidation or air oxidation needs longer reaction time and results in low yield. Further, in large scale, the conversion of dithiol to disulfide bond ends in unconverted starting material.
Another solid phase approach describes in Bioconjugate chem. 2009, 20, 1323-1331. This article discloses the process of somatostatin and octreotide analogues using solid phase peptide synthesis with CTC resin.
Journal of Harbin Institute of Technology, 2008, Vol 40 (2), 292-295, discloses the process for the preparation of octreotide using CTC resin. According to this process the obtained octreotide has the purity 70.26% by HPLC. During the process of peptide bond formation which is mediated by a coupling agent, the carboxylic group of amino acid interacts with the coupling agent to form an activated intermediate, which in turn interacts with the amino group of the next amino acid.
Racemization is a side-reaction that occurs during the preparation of a peptide. In large scale production, the formations of small amounts of epimers are possible. Detection and removal of these impurities are very difficult. This constitutes one of the most serious drawbacks for the implementation of peptides in commercial scale production.
Conventional syntheses of OCT may be divided two main approaches, direct solid-phase synthesis and liquid-phase synthesis. Direct solid-phase synthesis comprises attachment of a C-terminal amino acid to a resin, and step-by step elongation of the peptide chain, with pre- activated amino acids.
This route is expensive because it requires large excesses of starting amino acids and additionally is quite labor consuming as the peptide size increases, necessitating complex purification procedures to separate the product from the impurities since they are very similar to the final product. These shortcomings are especially important for large scale industrial production of the product. For example, see Canadian Patent Application 2,309,312 and U.S. Patent No. 6,476,186. With each successive condensation reaction required to add an amino acid, waste of starting materials increases, and purification steps are repeated. Liquid-phase synthesis comprises condensation of amino acids in solution. Several blocks, containing from 2 to 5 amino acids may be synthesized independently, followed by condensation of these synthons to each other in the required sequence.
For example, see WO 03/097668; U.S. Patent No. 4,395,403; and RU 2196144 C1. The advantage of this kind of processes is that it is less expensive than the previous one and the product is easier to purify. This method is also more effective for scale-up. However, liquid phase synthesis of lengthy peptide blocks, for example having more than 3 amino acids, is inefficient. Liquid-phase octreotide synthesis has the drawback is that the method is extremely labor-intensive and time consuming.
U.S. Patent No. 6,346,601 describes a method for octreotide synthesis where a solid-phase method is used to obtain a 7-mer, followed by condensation in solution with the modified amino acid threoninol. However, by using solid- phase synthesis to produce a 7-mer, only one less condensation is required compared to the solid-phase process for forming octreotide itself. Thus, only a marginal efficiency is introduced.
Summary of the invention According to an embodiment of the invention, there is provided a process for obtaining octreotide or a pharmaceutically acceptable salt thereof by hybrid solid-phase – liquid-phase synthesis. The synthesis comprises the steps of condensing two or three peptide blocks using liquid phase condensation to form a condensation product followed by cyclizing the product.
Each peptide block contains two or more amino acid residues, and at least one of the blocks is synthesized by solid-phase synthesis. The condensation product comprises in sequence the amino acids residues of octreotide. In the step of cyclizing, the condensation product is cyclized to form a disulfide bridge between the two cysteine residues, thereby forming octreotide. Further, according to another embodiment of the invention, a process is provided for obtaining an intermediate in octreotide synthesis by hybrid solid- phase – liquid-phase synthesis.
The synthesis of the intermediate comprises the steps of obtaining two or three peptide blocks, each peptide block containing two or more amino acid residues, and at least one of the blocks is synthesized by solid-phase synthesis. Subsequently, the peptide blocks are condensed using liquid phase condensation to form a condensation product, wherein the condensation product comprises in sequence the amino acids residues of octreotide.
This invention provides a more cost-effective and labor-saving method for obtaining OCT and its pharmaceutically acceptable salts by means of hybrid solid-phase – liquid-phase synthesis. The invention involves liquid phase condensation of two peptide blocks, at least one of which is obtained by solid- phase synthesis, the blocks containing more two or more amino acid residue in every block, followed by formation of a disulfide bridge from the two cysteine groups. Optionally, three blocks may be condensed. This hybrid solid phase-liquid phase method involves formation of one or more blocks of the octreotide amino acid sequence by solid-phase synthesis, followed by liquid phase condensation of the block(s) with required supplementary amino acids or other block(s) of amino acids.
This method is a blend of solid-phase and liquid-phase synthesis methods, combining the efficiencies of preparing shorter (6-mer or less) peptides using a solid-phase method with relative cheapness and easiness of purification of the product, characteristic of the liquid-phase method. Generally, the methods of invention comprise synthesizing specific side- chain protected peptide fragment intermediates of OCT on a solid support or in solution, coupling of the protected fragments in solution to form a protected OCT, followed by deprotection of the side chains and oxidation to yield the final OCT. The present invention further relates to individual peptide fragments which act as intermediates in the synthesis of the OCT
………………
Stage-I: Preparation of protected octreotide anchored to 2-CTC Resin
Method -1:
Octreotide was synthesized manually on 2-chlorotrityl chloride resin (substitution 0.90 mmol/g) by standard Fmoc solid phase synthesis strategy. The resin was soaked in the mixture of DC and DMF for the swelling. Fmoc-Thr(tBu)-OL was treated with the swelled 2- CTC resin in DCM in the presence of DIEA and substitution level was determined by weight gain measurements and also by UV Method. After the coupling of the first amino acid onto the resin, the un-reacted linkers on the resin (polymer) are protected, to avoid the undesired peptide chain formation, with a solution of 5% DIEA and 10% methanol in DCM. This process of capping is performed after anchoring the first protected amino acid to the resin. The complete synthesis was achieved by stepwise coupling of Fmoc-Amino acids to the growing peptide chain on the resin. All the couplings were carried out in DMF. The N- terminal Fmoc group was removed with 20 %( V/V) piperidine in DMF. The couplings were performed by dissolving the Fmoc-Amino acid (2 eq.) and HOBt (2 eq.) in DMF. The solution was cooled on ice and then DIC (2 eq.) was added. The reaction mixture was added to the resin and allowed to react for 2 hrs. The efficiency of the coupling was monitored using the Kaiser Ninhydrin test. The coupling step was repeated if Kaiser test was found positive. The sequence of addition for the synthesis of Octeriotide was Fmoc-Cys(Trt), Fmo-Thr(tBu), Fmoc-Lys(Boc), Fmoc-Trp(Boc), Fmoc-Phe, Fmoc-Cys(Trt), Boc-D-Phe.
Method -2:
Octreotide was synthesized manually on 2-chlorotrityl chloride resin (substitution 0.90 mmol/g) by standard Fmoc solid phase synthesis strategy. The resin was soaked in the mixture of MDC and DMF for the swelling. Fmoc-Thr-OL was treated with the swelled 2-CTC resin in DCM in the presence of DIEA and substitution level was determined by weight gain measurements and also by UV Method. After the coupling of the first amino acid onto the resin, the un-reacted linkers on the resin (polymer) are protected, to avoid the undesired peptide chain formation, with a solution of 5% DIEA and 10% methanol in DCM. This process of capping is performed after anchoring the first protected amino acid to the resin. The complete synthesis was achieved by stepwise coupling of Fmoc-Amino acids to the growing peptide chain on the resin. All the couplings were carried out in DMF. The N- terminal Fmoc group was removed with 20 %( V7V) piperidine in DMF. The couplings were performed by dissolving the Fmoc-Amino acid (2 eq.) and HOBt (2 eq.) in DMF. The solution was cooled on ice and then DIC (2 eq.) was added. The reaction mixture was added to the resin and allowed to react for 2 hrs. The efficiency of the coupling was monitored using the Kaiser Ninhydrin test. The coupling step was repeated if Kaiser test was found positive. The sequence of addition for the synthesis of Octeriotide was Fmoc-Cys(Trt), Fmo-Thr(tBu), Fmoc-Lys(Boc), Fmoc-Trp(Boc), Fmoc-Phe, Fmoc-Cys(Trt), Boc-D-Phe.
Stage-ll: Cleavage of peptide from resin along with global deprotection
The peptide resin (200 g, obtained in stage I) was swelled in DCM (500 mL) for 15 to 20 minutes under nitrogen at 25-30° C. The cocktail mixture (2.0 L – TFA (1.8 L), water (80 mL) DCM (80mL) and TIPS (80 mL)) was charged to the resin at 25-30° C. and the obtained reaction mixture was stirred for 2.5 hours at 25-30°C under nitrogen atmosphere. The reaction mixture was filtered and washed the resin with TFA (250 mL). The obtained filtrate was charged into cold MTBE (4 L, pre-cooled to a temperature of 0 -5° C) under stirring and allowing the temperature to rise more than 5° C. The reaction mixture was stirred for 45-75 minutes at 0-5°C. The obtained suspension was filtered, washed the solid with MTBE (5 L) and dried the solid under nitrogen. The product was stir with 5%ethanol in ethyl acetate at 25-30°C. Filtered the product, wash ith ethyl acetate and dried under vacuum to obtain a desired product
Stage-Ill: Disulphide bridge formation
The free thiol (100 g) obtained above is dissolved in methanol (22.0 L) with small amount of acetic acid and water (4.5 L) and stirred. Iodine solution (20gm iodine in 500 mL methanol) was added to the reaction mass slowly up to yellow color persists. The reaction was maintained for another 2 hrs, and the excess iodine quenched with Indion 830-S Resin (900 g) and filtered the resin. The filtrate was evaporated and precipitated using TBE or directly taken the solution for purification using preparative HPLC.
Stage -IV: Preparative HPLC Purification
Method-1 :
The crude disulphide bridge peptide was purified on a preparative reverse phase HPLC system using Kromasil C-18, 10 micron (50 x 250 mm). and eluting with a solvent system of 0.2% acetic acid in water(A) and 0.2% acetic acid in methanol(B). A linear gradient of 20- 60% B was used at a flow rate of 80mlJmin and detection at 220 nm.
The octreotide was eluted at around 25% methanol. The fractions were collected at regular intervals and assayed by HPLC to determine the purity of fractions. The desired purities fractions were pooled together and evaporated using Rota evaporator. The aqueous layer was lyophilized to isolate octreotide acetate
Method-2:
The crude disulphide bridge peptide was purified on a preparative reverse phase HPLC system using Kromasil C-18, 10 micron (50 x 250 mm) and eluting with a solvent system of 0.4% acetic acid in water(A) and methanol(B). A linear gradient of 25-60% B was used at a flow rate of 80mL/min and detection at 220 nm.
The octreotide was eluted at around 25% methanol. The fractions are collected at regular intervals and are assayed by HPLC to determine the purity and fractions. The desired purities may be pooled together and were evaporated using Rota evaporator. The aqueous layer was lyophilized to isolate octreotide acetate >
……………………….
Octreotide is a highly potent and pharmacologically selective analog of somatostatin. It inhibits growth hormone for long duration and is thereof indicated for acromegaly to control and reduce the plasma level of growth hormone. The presence of D-Phe at the N-terminal and an amino alcohol at the C-terminal, along with D-Tryptophan and a cyclic structure makes it very resistant to metabolic degradation.
Octreotide comprises 8 amino acids which has the following structural formula:
(D)Phe-Cys-Phe-{D)Trp-Lys-Thr-Cys-Thr-OL
Formula(l) wherein sulphur atoms of the Cys at the position 2 and of the Cys at the position 7 are mono-cyclic to form an -S-S- bridge.
A considerable number of known, naturally occurring small and medium-sized cyclic peptides as well as some of their artificial derivatives and analogs possessing desirable pharmacological properties have been synthesized. However, wider medical use is often hampered due to complexity of their synthesis and purification. Therefore, improved methods for making these compounds in simple, lesser steps and at lesser cost are desirable and this is the felt need of the industry and the mankind.
Conventional synthesis of octreotide may be divided into two main approaches, direct solid-phase synthesis and liquid-phase synthesis. Solution phase synthesis has been described by Bauer et al., (Sandoz) (Eur. Pat. Appl. 29,579 and U.S. Pat. No. 4,395,403). The process comprises: removing protected group from peptide; linking together by an amide bond two peptide unit; converting a function group at the N- or C-terminal; oxidizing a straight chain polypeptide by boron tristrifluoroacetate. This process involves a time-consuming, multi-step synthesis, and it is difficult to separate octreotide from the reaction mixtures since all the synthesis steps are carried out in liquid phase.Another solution phase approach described by Chaturvedi, et al., (Wockhardt) in U.S. Pat. No. 6,987,167 and EP 1506219 A, claims the cyclization of partially deprotected octreotide in the solution phase using iodine under conditions and for a time sufficient to form the octreotide.
Synthesis in solid phase have been described subsequently (Mergler et al., Alsina et al., Neugebauer). The above prior art for solid phase peptide synthesis cites the octapeptide formation, by starting the synthesis from the threoninol residue which makes it mandatory to protect this residue. Mergler et al., (Peptides: Chemistry and Biology. Proceedings of the 12* American Peptide Symposium. Smith, J.A. And Rivier J.E. Eds ESCOM, Leiden, Poster 292 Presentation, (1991) ) describes a synthetic process, using an aminoethyl resin upon which the Threoninol residue is incorporated with the two alcohol functions protected in acetal form The synthesis is carried out following an Fmoc/tBu protection scheme, forming the disulphide bridge on resin by oxidation of the thiol groups of the previously deprotected cysteine residues and releasing and deprotecting the peptide with a 20% mixture of TFA/DCM.
In early 1997, Alsina J. et al. ( Alsina J., Chiva C, Ortiz M., Rabanal F., Giralt E., and Albericio F., Tetrahedron Letters, 38, 883-886, 1997) described the incorporation, on active carbonate resins, of a Threoninol residue with the amino group protected by the Boc group and the side chain protected by a BzI group. The synthesis was then continued by Boc/Bzl strategy. Formation of the disulfide bridge was carried out directly on resin using iodine and the peptide was cleaved from the resin and its side chain protecting groups were simultaneously removed with HF/anisole 9/1. At the final stage the formyl group was removed with a piperidine/DMF solution.
Neugebauer (Neugebauer W., Lefevre M.R., Laprise R, Escher E., Peptides: Chemistry, Structure and Biology, p 1017, Marshal G.R. And Rivier J.E. Eds. ESCOM.Leiden (1990) described a linear synthesis with a yield of only 7%.
Edwards et al., (Edwards B.W., Fields C.G., Anderson CJ., Pajeau T.S., Welch M.J., Fields G.B., J.Med.Chem. 37, 3749-3757 (1994) carried out another another solid- phase type approximation; they synthesized step-by-step on the resin, the peptide D- Phe-Cys(Acm)-Phe-D-Tφ(Boc)-Lys(Boc)-Thr(tBu)-Cys(Acm)-HMP-Resin. Next they proceeded to form the disulfide on resin and then release the peptide from the resin by means of aminolysis with threoninol, with obtaining a total yield of only 14%.
The solid phase synthesis described by Yao-Tsung Hsieh et. al., in U.S. Pat. No. 6,476,186 involves the synthesis of octreotide by using Thr(ol)(tBu)-2Cl-trityl resin as starting material followed by the cleavage of the straight chain peptide from the resin by using a strong acid and the formation of the intra-molecular disulfide bond on the completely deprotected octreotide by oxidation using charcoal catalyst and a higher yield of >70%.
Another solid phase synthesis described by Berta Ponsati et.al (Lipotec) in U.S. Pat No. 6,346,601 and EP 0953577 B involve the coupling of threoninol on the protected heptapeptide in solution, after a selective acid cleavage from the chlorotrityl resin without affecting the peptide side-chain protecting groups.
A hybrid solid phase-liquid phase method for synthesis of octreotide described by Iarov et al., (Dalton Chemical Laboratories) in WO 2005087794 wherein the method comprises liquid phase condensation of two or three peptide blocks in which at least one peptide block is synthesized by solid-phase method.
EP 1511761 Bl involves cyclization on the semi-protected linear peptide wherein one of the cysteine residue is protected with an orthogonal protecting group. The radioactive isotope labeling of octreotide by the coupling of bifunctional chelating agents like DTPA or DOTA to the peptide was described by Te- Wei Lee et al., in U.S. Pat. No. 5,889,146 (Inst, of Nuclear Energy Research)
The method for cyclization of linear vapreotide by means of intramolecular cysteine formation has been described by Quattrini et. al., (Lonza AG) in WO 2006048144, wherein the process involves the synthesis of linear vapreotide peptide on Sieber-resin (from Novabiochem) by Fmoc standard groups, wherein the side chain protecting groups are D or L-Trp(Boc), Cys(Trt), Lys(Boc), Tyr(tBu). The protected peptide is cleaved off in 5% TFA in dichloromethane and then globally deprotected by acidolysis in a cleavage mix of 300 equivalents of concentrated TFA, 12 equivalents of Dithiothreitol, 12 equivalents of Dichloromethane, 50 equivalents of water forl hour at room temperature. The Boc groups are removed. The product was subjected to charcoal method using trace amounts of activated, powdered charcoal wherein a concentration of the linear cysteinyl peptide of 50 mg/ml (1 eq.) in DMF in the presence of 1 eq. Diisopropyl-ethyl-amine and that additionally air was sparged at low pressure into the liquid under stirring. After 15-20 hrs, 100% conversion was achieved with 84% (w/w) analytical yield of 79% vapreotide.
The formation of intramolecular disulphide formation in a polypeptide by reacting with hydrogen peroxide has been described by Mineo Niwa et al. (Fujisawa Pharmaceutical Co.) in U.S. Pat. No.5, 102,985 wherein the reaction is to be carried out at a pH of about 6 tol 1, wherein the molar ratio of H2O2 to polypeptide is within the range of 1:1 to 100:1. The above cited prior art mainly carries out the cyclization of the peptide on the resin or on partially protected or protected peptides. The use of partial or minimal protecting group strategies and improvement in the activation methods have considerable effect on limitations of poor solubility and possible danger of racemization due to the overactivation of carboxyl groups. However, these approaches do not overcome the problem of the poor coupling efficiency between large peptide segments, because of the intrinsic difficulty of obtaining effective molar concentrations for high molecular weight molecules.
Example 8:
Oxidation of S-H peptide with DMSO-HCl to get S-S peptide:
(D)Phe-Cys-Phe-(D)Trp-Lys-Thr-Cys-Thr-OL
Formula (1)
S-H peptide ( 9g) was dissolved in 6.5L DMSO and under ice-cooling 6.5L IM HCl was added slowly so that temperature is below 26°C. Stirring was continued for 6 hours. At room temperature after six hours reaction mixture was diluted with 13L of water and filtered through Whatman no. 41 through Celite bed. The filtrate was loaded on C- 18 column for concentration. The compound was eluted with 100% acetonitrile. The eluant was concentrated on rotavap and then the concentrated solution was centri-evaporated to dryness. The RP-HPLC profile of crude octreotide is depicted in Figure 1.
Weight of crude peptide =3.9g.(45%)
Purity: 44.25%
Example 9:
Purification of crude octreotide:
The crude octreotide was loaded on to cation ion exchange column and eluted using a salt gradient using a Akta Purifier (by Amersham, Sweden) low pressure chromatography system. The IEX fractions of purity >70% were further loaded for RP-HPLC purification on Kromacil C-18 column of (250x50mm,100A°.) The peptide was purified by using aqueous TF A(O-0.5%) and methanol/ethanol and/or Acetonitrile in a gradient program on a Shimadzu preparative HPLC System consisting of a controller, 2 LC8A pumps, and UV-Vis detector. The purified peptide was analysed by analytical RP-HPLC (Figure 5). Fractions of > 99% purity were subjected either by RP-HPLC or IEX to salt exchange and concentrated to remove organic solvent either by rota or reverse osmosis and subsequently lyophilized to get final API with purification step yield of 70% or above.The MS spectrum of octreotide is depicted in Figure 6.
References
- Official manufacturer website for up-to-date dosing & safety information:http://www.sandostatin.com
- Maurer R, Gaehwiler BH, Buescher HH, Hill RC, Roemer D. Opiate antagonistic properties of an octapeptide somatostatin analog. Proceedings of the National Academy of Sciences USA. 1982 Aug;79(15):4815-7. PMID 6126877
- Allen MP, Blake JF, Bryce DK, Haggan ME, Liras S, McLean S, Segelstein BE. Design, synthesis and biological evaluation of 3-amino-3-phenylpropionamide derivatives as novel mu opioid receptor ligands. Bioorganic and Medicinal Chemistry Letters. 2000 Mar 20;10(6):523-6.PMID 10741545
- Medscape: Octreoscan review
- Joshua Chin, Matthew Vesnaver, Vadim Bernard-Gauthier, Erin Saucke-Lacelle, Björn Wängler, Carmen Wängler, Ralf Schirrmacher. Amino Acids: Direct one-step labeling of cysteine residues on peptides with 11C-methyl triflate for the synthesis of PET radiopharmaceuticals. Amino Acids. 2013 Aug 7. PMID 23921782
- Lustig RH, Hinds PS, Ringwald-Smith K, Christensen RK, Kaste SC, Schreiber RE, Rai SN, Lensing SY, Wu S, Xiong X (June 2003). “Octreotide therapy of pediatric hypothalamic obesity: a double-blind, placebo-controlled trial”. J. Clin. Endocrinol. Metab. 88 (6): 2586–92.doi:10.1210/jc.2002-030003. PMID 12788859.
- Flier JS (2004). “Obesity wars: Molecular progress confronts an expanding epidemic”. Cell116 (2): 337–50. doi:10.1016/S0092-8674(03)01081-X. PMID 14744442.
- Boulpaep, Emile L.; Boron, Walter F. (2003). Medical physiologya: A cellular and molecular approach. Philadelphia: Saunders. p. 1227. ISBN 0-7216-3256-4.
- Lustig RH (2011). “Hypothalamic obesity after craniopharyngioma: mechanisms, diagnosis, and treatment”. Front Endocrinol (Lausanne) 2: 60. doi:10.3389/fendo.2011.00060.PMC 3356006. PMID 22654817.
- Lustig RH, Greenway F, Velasquez-Mieyer P, Heimburger D, Schumacher D, Smith D, Smith W, Soler N, Warsi G, Berg W, Maloney J, Benedetto J, Zhu W, Hohneker J (February 2006). “A multicenter, randomized, double-blind, placebo-controlled, dose-finding trial of a long-acting formulation of octreotide in promoting weight loss in obese adults with insulin hypersecretion”. Int J Obes (Lond) 30 (2): 331–41. doi:10.1038/sj.ijo.0803074.PMC 1540404. PMID 16158082.
- Uhl W, Anghelacopoulos SE, Friess H, Büchler MW (1999). “The role of octreotide and somatostatin in acute and chronic pancreatitis”. Digestion. 60 Suppl 2: 23–31.doi:10.1159/000051477. PMID 10207228.
- Shima Y, Ohtsu A, Shirao K, Sasaki Y (May 2008). “Clinical efficacy and safety of octreotide (SMS201-995) in terminally ill Japanese cancer patients with malignant bowel obstruction”.Jpn. J. Clin. Oncol. 38 (5): 354–9. doi:10.1093/jjco/hyn035. PMID 18490369.
- Skagen C, Einstein M, Lucey MR, Said A (Feb 2009). “Combination Treatment With Octreotide, Midodrine, and Albumin Improves Survival in Patients With Type 1 and Type 2 Hepatorenal Syndrome.”. J Clin Gastroenterol. 43 (7): 680–5. doi:10.1097/MCG.0b013e318188947c.PMID 19238094.
- Patient.co.uk (Feb 2013). Hypotension.
- Kilic D, Sahin E, Gulcan O, Bolat B, Turkoz R, Hatipoglu A (2005). “Octreotide for treating chylothorax after cardiac surgery”. Tex Heart Inst J 32 (3): 437–9. PMC 1336729.PMID 16392238.
- Siu SL, Lam DS (2006). “Spontaneous neonatal chylothorax treated with octreotide”. J Paediatr Child Health 42 (1-2): 65–7. doi:10.1111/j.1440-1754.2006.00788.x.PMID 16487393.
- Chan EH, Russell JL, Williams WG, Van Arsdell GS, Coles JG, McCrindle BW (November 2005). “Postoperative chylothorax after cardiothoracic surgery in children”. Ann. Thorac. Surg. 80(5): 1864–70. doi:10.1016/j.athoracsur.2005.04.048. PMID 16242470.
- Gøtzsche PC, Hróbjartsson A (2008). “Somatostatin analogues for acute bleeding oesophageal varices”. Cochrane Database Syst Rev (3): CD000193.doi:10.1002/14651858.CD000193.pub3. PMID 18677774.
- Greek Researchers Investigate Octreotide Hypertension Research Foundation, accessed 2011-01-02
- Panagopoulos GN, Deftereos SN, Tagaris GA, Gryllia M, Kounadi T, Karamani O, Panagiotidis D, Koutiola-Pappa E, Karageorgiou CE, Piadites G (2007). “Octreotide: a therapeutic option for idiopathic intracranial hypertension”. Neurol Neurophysiol Neurosci: 1. PMID 17700925.
- Haberfeld, H, ed. (2009). Austria-Codex (in German) (2009/2010 ed.). Vienna: Österreichischer Apothekerverlag. ISBN 3-85200-196-X.
- ^ Jump up to:a b c d Dinnendahl, V, Fricke, U, ed. (2010). Arzneistoff-Profile (in German) 8 (23 ed.). Eschborn, Germany: Govi Pharmazeutischer Verlag. ISBN 978-3-7741-9846-3.
- Hovind P, Simonsen L, Bülow J (March 2010). “Decreased leg glucose uptake during exercise contributes to the hyperglycaemic effect of octreotide”. Clin Physiol Funct Imaging 30(2): 141–5. doi:10.1111/j.1475-097X.2009.00917.x. PMID 20132129.
- Saif MW (July 2011). “Rheumatoid arthritis associated with the use of Sandostatin® LAR® depot in a patient with pancreatic neuroendocrine tumor. An association or a coincidence? The first case report”. JOP 12 (4): 425–8. PMID 21737909. Lay summary – eHealthMe.com.
- van der Lely AJ, de Herder WW, Lamberts SW (November 1997). “A risk-benefit assessment of octreotide in the treatment of acromegaly”. Drug Saf 17 (5): 317–24. PMID 9391775.
- Kapicioglu S, Mollamehmetoglu M, Kutlu N, Can G, Ozgur GK (January 1998). “Inhibition of penile erection in rats by a long-acting somatostatin analogue, octreotide (SMS 201-995)”. Br J Urol 81 (1): 142–5. PMID 9467491.
- Klopp, T, ed. (2010). Arzneimittel-Interaktionen (in German) (2010/2011 ed.). Arbeitsgemeinschaft für Pharmazeutische Information. ISBN 978-3-85200-207-1.
| US8507432 | Jun 11, 2010 | Aug 13, 2013 | Endo Pharmaceuticals Solutions Inc. | Controlled release formulations of octreotide |
| US20100247594 * | Jun 11, 2010 | Sep 30, 2010 | Endo Pharmaceuticals Solutions Inc. | Delivery of dry formulations of octreotide |
| US20110009338 * | Jun 11, 2010 | Jan 13, 2011 | Endo Pharmaceuticals Solutions Inc. | Controlled release formulations of octreotide |
| WO2010089757A2 | May 4, 2009 | Aug 12, 2010 | Usv Limited | An improved process for synthesis of cyclic octapeptide |
| WO2013046233A2 | Sep 28, 2012 | Apr 4, 2013 | Mylan Laboratories Ltd | Process for the preparation of octreotide acetate |
| WO2013132505A1 | Mar 9, 2012 | Sep 12, 2013 | Natco Pharma Limited | Improved process for preparation of octreotide by solution phase peptide synthesis |
| US8377891 | May 4, 2009 | Feb 19, 2013 | Usv, Ltd. | Process for synthesis of cyclic octapeptide |
| WO2003097668A2 * | Apr 16, 2003 | Nov 27, 2003 | Suresh Beri | Novel process for production of the somatostatin analog, octreotide |
| US6346601 * | Jan 29, 1999 | Feb 12, 2002 | Lipotec S.A. | Procedure for obtaining the somatostatin analog, octreotide |
| US6476186 * | May 24, 2000 | Nov 5, 2002 | Institute Of Nuclear Energy Research | Process for preparing octreotide and derivatives thereof |
| WO2005087794A1 | Mar 14, 2005 | Sep 22, 2005 | Dalton Chemical Lab Inc | Process for octreotide synthesis |
| WO2007110765A2 | Mar 28, 2007 | Oct 4, 2007 | Deshpande Amol Ashok | Processes for the preparation of octreotide |
| WO2010089757A2 | May 4, 2009 | Aug 12, 2010 | Usv Limited | An improved process for synthesis of cyclic octapeptide |
| US4395403 | Nov 16, 1981 | Jul 26, 1983 | Sandoz Ltd. | Polypeptides, processes for their production, pharmaceutical compositions comprising said polypeptides and their use |
| US6346601 | Jan 29, 1999 | Feb 12, 2002 | Lipotec S.A. | Procedure for obtaining the somatostatin analog, octreotide |
| US6476186 | May 24, 2000 | Nov 5, 2002 | Institute Of Nuclear Energy Research | Process for preparing octreotide and derivatives thereof |
| US6987167 | May 22, 2002 | Jan 17, 2006 | Wockhardt Limited | Process for production of the somatostatin analog, octreotide |
| US20040039161 | Aug 22, 2002 | Feb 26, 2004 | Mayer John Philip | Use of trichloroacetimidate linker for peptide synthesis |
















Sorry, the comment form is closed at this time.