cas no 116684-92-5
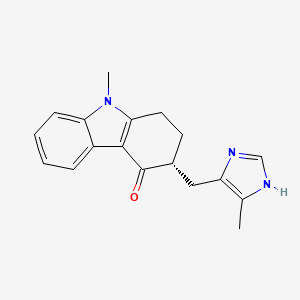
(3R)-9-methyl-3-[(5-methyl-1H-imidazol-4-yl)methyl]-2,3-dihydro-1H-carbazol-4-one
Molecular Formula: C18H19N3O Molecular Weight: 293.36296
GR-81225X
GR-82115C (hydrochloride)
GSK
PRECLINICAL
Nausea and Vomiting, Treatment of
| CA 2081709 EP 0542364 US 4859662 |
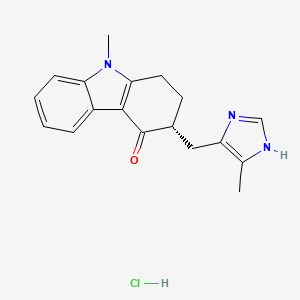
GALDANSETRON HYDROCHLORIDE
CAS NO 156712-35-5 (HCl)
Molecular Formula: C18H20ClN3O Molecular Weight: 329.8239
- Galdansetron HCl
- Galdansetron hydrochloride
- GR 81225C
- GR 81225X [as the base]
- UNII-E3M2R8Q947

GALDANSETRON RACEMIC
………………………………………………………………
Patents
- US 20050209293 A
- US 20060024365
- US 4859662 A
- DE 3740352 A1
- WO 2001095902 A1
- WO 2009146537 A1
- US 20100226943 A1

Example 1
(E) -1,2,3,9-tetrahydro-9-methyl-3-[(5-methyl-1H-imidazol-4-yl) methylene]-4H-carbazol-4-one maleate
A solution of 1,2,3,9-tetrahydro-3-[hydroxy [5-methyl-1-(triphenylmethyl)-1H-imidazol-4-yl] methyl]-9-methyl-4H-carbazol-4-one (2.70 g) in glacial acetic acid (100 ml) was treated with p-toluenesulfonic acid monohydrate (10.80 g) and the stirred solution was heated to reflux for 4 hours. The cool dark liquid was evaporated, treated with aqueous saturated sodium bicarbonate solution (250 ml) and extracted into ethyl acetate (4 × 250 ml). The combined, dried organic extracts were evaporated and purified by SPCC. The eluting with System A (978: 20: 2 → 945: 50: 5) afforded the free base of the title compound as a light yellow-brown solid (488 mg). A hot solution of the free base (87 mg) in ethanol, about 16 ml) was treated with a hot solution of maleic acid (38 mg) in ethanol (1 ml). After cooling, the precipitate was collected to give the title compound (81 mg), mp 205-209 ° was obtained.
Analysis found: C: 65.1, H 5.2, N 10.2; C ₁ ₈ H ₁ ₇ N ₃ O · C ₄ H ₄ O ₄
theoretical values: C: 64.9, H 5.2, N 10.3%.
Example 7 1,2,3,9-tetrahydro-9-methyl-3-[(1H-imidazol-4-yl) methylene] – 4H-carbazol-4-one
A solution of diisopropylamine (1.54 ml) in dry THF (20 ml) of -78 ° was treated dropwise with n-butyllithium (1.32 M in hexane, 8.3 ml). The mixture was allowed to warm to 0 ° and cooled to -78 ° again. It was then in the course of 3 minutes to a stirred suspension of 1,2,3,9-tetrahydro-9-methyl-4H-carbazol-4-one (2.0 g) in dry THF (80 ml) of -78 optionally °. The resulting suspension was stirred at -78 ° on this for 2 hours and then treated with 1 – treated (triphenylmethyl)-1H-imida zol-4-carboxaldehyde (3.72 g). The mixture was stirred for a further 2 hours, during which time it was allowed to warm slowly to room temperature. Then it was cooled to -78 ° and quenched with acetic acid (2 ml). The resulting solution was allowed to warm to room temperature and 8% aqueous sodium bicarbonate solution (600 ml) was poured.
The mixture was extracted with dichloromethane (3 x 150 ml) and the combined, dried organic extracts were evaporated to give a foam. A solution of this foam and p-toluenesulfonic acid monohydrate (18 g) in a mixture of glacial acetic acid (25 ml) and dry THF (150 ml) was heated for 5 hours under reflux. The cooled mixture was carefully added to an 8% aqueous sodium bicarbonate solution (650 ml) and extracted with dichloromethane (3 x 150 ml). The combined, dried organic extracts were evaporated to give a solid which was obtained by FCC eluting with System A (100: 1: 10) was purified. In this way the title compound (1.42 g), mp 225-232 ° was obtained.
Analysis found: C: 73.3, H 5.6, N 14.7; C ₁ ₇ H ₁ ₅ N ₃ O
theoretical values: C: 73.6, H 5.5, N 15.1%
Example 8
1,2,3,9-tetrahydro-9-methyl-3-[(5-methyl-1H-imidazol-4-yl) methyl]-4H-carbazol-4-one maleate
A solution of 1,2,3,9-tetrahydro-9-methyl-3-[(5-methyl-1H-imidazol-4-yl) methylene]-4H-carbazol-4-one (3.50 g) in DMF (85 ml) and ethanol (50 ml) was added to a prereduced suspension of 10% palladium on carbon added (3.4 g) in ethanol (50 ml) and hydrogenated at room temperature and atmospheric pressure until hydrogen uptake ceased (270 ml). The catalyst was filtered off and the filtrate was evaporated. The residue was adsorbed from methanol (170 ml) of SPCC-FCC silica and applied to a column. A gradient elution system A (967: 30: 3 → 912: 80: 8) afforded the free base of the title compound as a solid (2.32 g). A portion of this solid (500 mg) in hot ethanol (15 ml) was treated with a hot solution of maleic acid (224 mg) in ethanol (2 ml). Upon cooling, a precipitate was collected, the title compound (415 mg), mp 130.5-137 ° revealed. tlc (system A 200: 10: 1) 0.30.
Analysis found: C: 63.2, H 5.5, N 9.7; C ₁ ₈ H ₁ ₉ N ₃ O · C ₄ H ₄ O ₄ · 0.33 H ₂ O
theoretical values: C: 63.6, H 5.7, N 10.1%. Water sample found: 1.55% wt. / Wt. 0.33 mol H ₂ O ≡
¹ H-NMR (d ⁶-DMSO) δ 1.8-1.98 (1H, m), 2.1-2.25 (1H, m), 2.25 (3H, s), 2.68 to 2, 84 (2H, m), 2.85 to 3.3 (3H, m), 3.75 (3H, s), 6.0 (2H, s-maleate), 7.18-7.32 (2H, m), 7.57 (1H, brd), 8.03 (1H, brd), 8.88 (1H, s).
+FORM
Example 31
(+) -1,2,3,9-Tetrahydro-9-methyl-3-[(5-methyl-1H-imidazol-4-yl) methyl]-4H-carbazol-4-one
A solution of (±) -1,2,3,9-tetrahydro-9-methyl-3-[(5-me thyl-1H-imidazol-4-yl) methyl]-4H-carbazol-4-one (500 mg) in warm methanol (30 ml) was treated with a solution of (+) -2,3-bis [[(4-methylphenyl) carbonyl] oxy] butanedioic acid (690 mg) in methanol (10 ml), and The solution was allowed to stand for 3 days at 0 °. It was then filtered to give a solid remained which was recrystallized from methanol to give the desired salt (195 mg), mp 146-148 ° was obtained. A part of this salt (186 mg) was suspended (10 ml) in water and treated with potassium carbonate (79.2 mg) and the mixture was extracted with dichloromethane (2 x 40 ml). The combined, dried organic extracts were evaporated in vacuo to give the title compound (79.2 mg) as a solid, mp 230-232 ° stayed behind. [Α] = 49.7 ° (c = 0.41%, CHCl ₃).
– FORM
Example 32
(-) -1,2,3,9-Tetrahydro-9-methyl-3-[(5-methyl-1H-imidazol-4-yl) methyl]-4H-carbazol-4-one
A solution of (±) -1,2,3,9-tetrahydro-9-methyl-3-[(5-me thyl-1H-imidazol-4-yl) methyl]-4H-carbazol-4-one (500 mg) in warm methanol (30 ml) was added a solution of (- treated) -2,3-bis [[(4-methylphenyl) carbonyl] oxy] butanedioic acid (690 mg) in methanol (10 ml), and The solution was allowed to stand at 0 ° for 3 days. It was filtered, producing a solid remained which was recrystallized from methanol to give the desired salt (162 mg), mp 147-148 ° was obtained. This was suspended in water (15 ml) and treated with a solution of potassium carbonate (1 g in 10 ml water). The mixture was extracted with dichloromethane (2 x 30 ml). The combined, dried organic extracts were evaporated in vacuo to give the title compound (72.5 mg) as a solid, mp 230-232 ° stayed behind. [Α] = 48.4 ° (c = 0.44%, CHCl ₃).
Example 33
1,2,3,9-tetrahydro-9-methyl-3-[(5-methyl-1H-imidazol-4-yl) – methyl]-4H-carbazol-4-one
A solution of Intermediate 7 (190 mg) in dry DMF (1 ml) was added dropwise to a stirred suspension of sodium hydride, under nitrogen (52% dispersion in oil, 20 mg) in dry DMF (0.4 ml). After 15 minutes, iodomethane (0.027 ml) was added and the mixture was stirred for 1.5 hours. Water (20 ml) was added and the suspension was extracted with dichloromethane (3 x 10 ml). The combined, dried organic extracts were evaporated to give an oil (ca. 300 mg) in a mixture of THF (4 ml), acetic acid (4 ml) and water (4 ml) was dissolved. The mixture was heated at reflux for 1.5 hours. It was poured (20 ml) in saturated potassium carbonate solution and extracted with dichloromethane (3 x 10 ml). The combined, dried organic extracts were evaporated to give a semi-solid (ca 255 mg) was obtained by SPCC eluting with System A (200: 1: 10) was purified. In this way the title compound (7 mg) was obtained. The ¹ H NMR and tlc of this material were values with the corresponding values of the product of Example 8 in line.
Example 34
1,2,3,9-tetrahydro-9-methyl-3-[(5-methyl-1H-imidazol-4-yl) – methyl]-4H-carbazol-4-one
n-Butyllithium (1.45M in hexane; 2.07 ml) was added dropwise to a cold (-70 (20 ml) under nitrogen. The solution was allowed to reach 0 30 min, cooled to -70 solution of) 1,2,3,9-tetrahydro-9-methyl-4H-carbazol-4-one (500 mg) in dry THF (10 ml under nitrogen. Hexamethylphosphoramide (0.44 ml) was added and the mixture was allowed to reach 0 cooled to -70 4-(chloromethyl)-5-methyl-1-(triphenylmethyl) -1H-imidazole (936 mg) in dry THF (15 ml) was added and the mixture was allowed to reach ca. 20 sodium bicarbonate solution (100 ml) and extracted with dichloromethane (3 give a semi-solid which was treated with a mixture of acetic acid (10 ml), water (10 ml) and THF (10 ml) and heated at reflux for 1.5 h. The solution was poured into saturated potassium carbonate solution (100 mml) and extracted with dichloromethane (3 organic extracts were evaporated to give a solid (ca. 1.8 g) which was purified by SPCC eluting with System A (200:10:1) to give the title compound (17 mg). The .sup.1 H-n.m.r. and t.l.c. of this material were consistent with those obtained from the product of Example 8.
BREAKING OF MALEATE SALT
Example 35
1,2,3,9-tetrahydro-9-methyl-3-[(5-methyl-1H-imidazol-4-yl) methyl]-4H-carbazol-4-one
1,2,3,9-tetrahydro-9-methyl-3-[(5-methyl-1H-imidazol-4-yl) – methyl]-4H-carbazol-maleate (37 mg) was partitioned between 2 N sodium bicarbonate solution (10 ml) and chloroform (3 x 15 ml) separated. The combined, dried organic layers were evaporated to give the free base (26 mg) was obtained, which was dissolved at -10 ° under nitrogen in 10% aqueous THF (4 ml). To this stirred solution, a solution of 2,3-dichloro-5 ,6-dicyano-1 ,4-benzoquinone (49 mg) was added in dry THF (1.6 ml) was added dropwise, and the reaction mixture was added over 3 hours allowed to warm to 0 °. The solution was evaporated in vacuo and purified by FCC eluting with System A (94.5: 5: 0.5) to give the title compound (10 mg) was obtained as a solid.The ¹ H NMR and tlc values of this material were consistent with the corresponding values of the product of Example 8
INTERMEDIATE 7
Intermediate 7
1,2,3,9-Tetrahydro-3-[(5-methyl-1-(triphenylmethyl)-1H-imidazol-4-yl) methyl]-4H-carbazol-4-one
A solution of triphenylchloromethane (4.2 g) in dry DMF (40 ml) was added dropwise to a solution of 1,2,3,9 – tetrahydro-3-[(5-methyl-1H-imidazol-4-yl) methyl ]-4H-carbazol-4-one (3.5 g) and triethylamine (1.75 ml) in dry DMF (35 ml) under nitrogen.After stirring for 4 hours the mixture was poured (300 ml) in water and extracted with dichloromethane (3 x 100 ml). The combined extracts were washed with water (200 ml), dried and evaporated to give an oil (about 9 g) was obtained. This was purified by FCC eluting with System A (200: 1: 10) to give the title compound (4.57 g) as a foam, tlc (system A 200: 10: 1), Rf 0.32 was obtained.
Intermediate 8
4 – (chloromethyl)-5-methyl-1-(triphenylmethyl)-1H-imidazole
A solution of thionyl chloride (1.3 ml) in dry dichloromethane (10 ml) was added over 5 minutes to a stirred suspension of 5-methyl-1-(triphenylmethyl) – 1H-imidazol-4-methanol (5.0 g ) in a mixture of dichloromethane (100 ml) and dry DMF (2 ml) at 0 °. The mixture was stirred at 0 ° for 30 minutes and washed successively with 8% sodium bicarbonate (2 x 50 ml), water (50 ml), dried and evaporated in vacuo below 40 ° to give an oil (5 g) was obtained. This was dissolved in ether (100 ml), and the resulting solution was filtered through a silica pad which was eluted with ether (2 x 100 ml) further. The combined filtrates were evaporated below 40 °, whereby a foam was obtained which was triturated with cold hexane and filtered. There was thus obtained the title compound (4.2 g) as a solid, mp 133-135 °, was obtained
Intermediate 14
1,2,3,9-tetrahydro-9-methyl-3 [[5-methyl-1-(triphenylmethyl) – 1H-imidazol-4-yl] methyl]-4H-carbazol-4-one
A solution of triphenylchloromethane (286 mg) in dry DMF (10 ml) was added dropwise to a stirred solution of 1,2,3,9-tetrahydro-9-methyl-3-[(5-methyl-1H-imidazol-4 – optionally yl) methyl]-4H-carbazol-4-one (292 mg) and triethylamine (101 mg) in dry DMF (20 ml). The resulting solution was stirred for 3.5 hours at room temperature under nitrogen. The reaction mixture was then poured into water (100 ml) and the resulting suspension was extracted with dichloromethane (3 x 50 ml). The combined, dried organic extracts were adsorbed onto FCC silica, which was then applied to a column. FCC eluting with System A (150: 8: 1) afforded a solid which, by crystallization from dichloromethane: hexane (2: 1) was further purified to give the title compound (304 mg), mp 193 to 195 °, was obtained.
INTERMEDIATES
CAS 27387-31-1
- C13 H13 N O
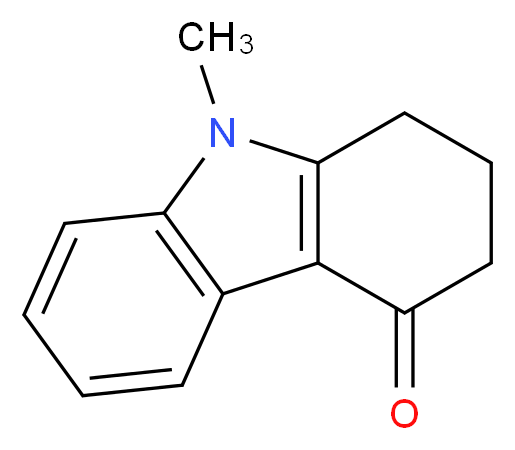
- 4H-Carbazol-4-one, 1,2,3,9-tetrahydro-9-methyl-
- Carbazol-4(1H)-one, 2,3-dihydro-9-methyl-
- INTERMEDIATE 2
- CAS 113140-81-1
-
- C24 H20 N2 O
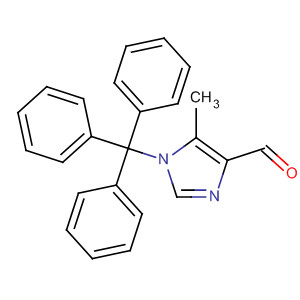
- 1H-Imidazole-4-carboxaldehyde, 5-methyl-1-(triphenylmethyl)
- INTERMEDIATE 3
- CAS : 116684-96-9
- C37 H33 N3 O2
- 4H-Carbazol-4-one, 1,2,3,9-tetrahydro-3-[hydroxy[5-methyl-1-(triphenymethyl)-1H-imidazol-4-yl]methyl]-9-methyl-
- 4H-Carbazol-4-one, 1,2,3,9-tetrahydro-3-[hydroxy[5-methyl-1-(triphenylmethyl)-1H-imidazol- 4-yl]methyl]-9-methyl-
- INTEMEDIATE 4
- triphenylchloromethane
- CAS 76-83-5
- INTERMEDIATE 5
- 4-(chloromethyl)-5-methyl-1-(triphenylmethyl) -1H-imidazole
- ……………………………………………………………………………………….


-

THANKS AND REGARD’S
DR ANTHONY MELVIN CRASTO Ph.DGLENMARK SCIENTIST , NAVIMUMBAI, INDIA
did you feel happy, a head to toe paralysed man’s soul in action for you round the clock
need help, email or call me
MOBILE-+91 9323115463web linkI was paralysed in dec2007
One Response to “GALDANSETRON”
Sorry, the comment form is closed at this time.













[…] GALDANSETRON » All About Drugs […]